Nedosiran
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kosar Doraghi, M.D.[2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Nedosiran is an LDHA-directed small interfering RNA that is FDA approved for the treatment of lower urinary oxalate levels in children 9 years of age and older and adults with primary hyperoxaluria type 1 (PH1) and relatively preserved kidney function, e.g., eGFR ≥ 30 mL/min/1.73 m2. Common adverse reactions include injection site reactions.
Adult Indications and Dosage
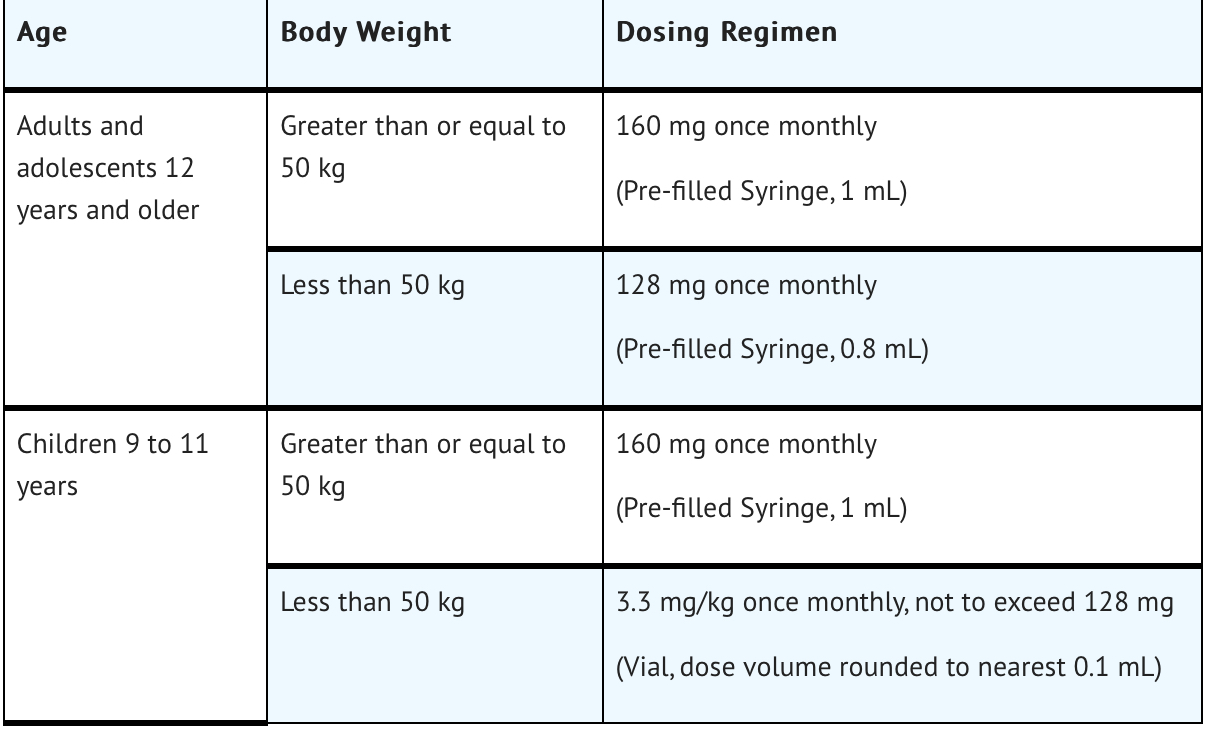
FDA-Labeled Indications and Dosage (Adult)
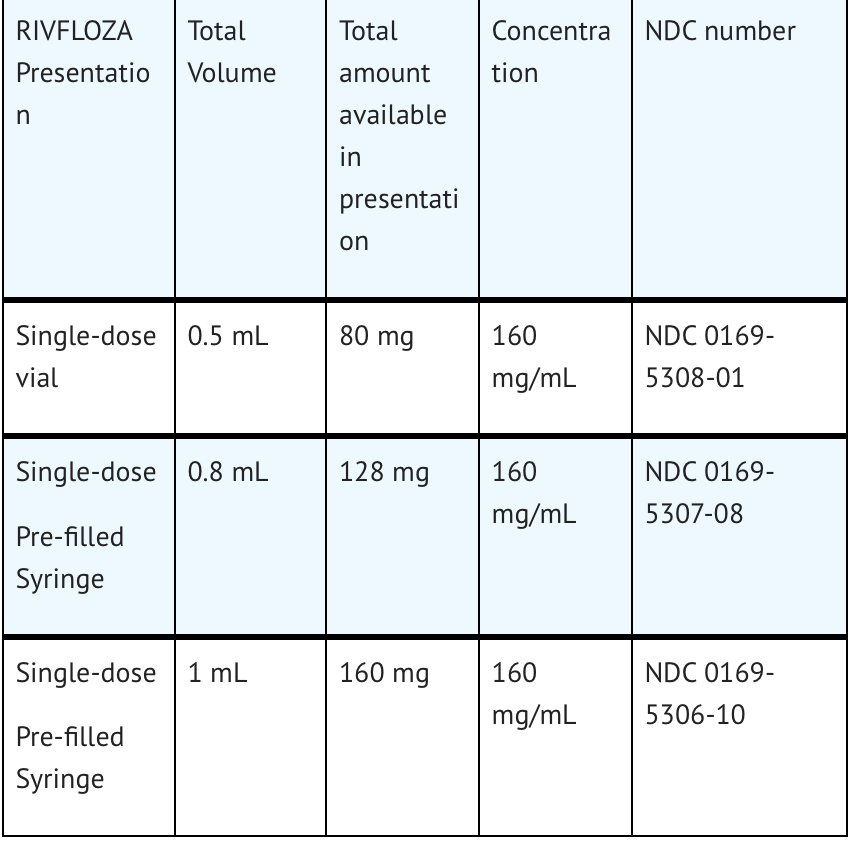
RIVFLOZA Injection 160 mg/mL is a clear, colorless-to-yellow solution available as follows:
- 80 mg (0.5 mL) single-dose vial
- 128 mg (0.8 mL) single-dose Pre-filled Syringe
- 160 mg (1 mL) single-dose Pre-filled Syringe
Off-Label Use and Dosage (Adult)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Nedosiran FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Contraindications
None.
Warnings
There is limited information regarding Nedosiran Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
- The safety of RIVFLOZA was evaluated in clinical trials involving 29 adults and 12 children with PH1 (primary hyperoxaluria type 1).
- Patients ranged in age from 9 to 46 years at the start of treatment, with a median exposure duration of approximately 15 months.
- In the placebo-controlled PHYOX2 trial, injection site reactions were the most *common adverse reaction, occurring in 39% of patients treated with RIVFLOZA compared to none on placebo. These reactions were generally mild and did not lead to treatment discontinuation.
- In the PHYOX3 extension study, which included 40 patients, atrophy at the injection site was reported in 3% of patients.
- It's important to note that adverse reaction rates observed in clinical trials may not reflect those observed in practice due to varying conditions.
Postmarketing Experience
There is limited information regarding Nedosiran Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Nedosiran Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Nedosiran in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Nedosiran in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Nedosiran during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Nedosiran in women who are nursing.
Pediatric Use
The safety and effectiveness of RIVFLOZA have been established in pediatric patients aged 9 years and older.
Geriatic Use
There is no FDA guidance on the use of Nedosiran in geriatric settings.
Gender
There is no FDA guidance on the use of Nedosiran with respect to specific gender populations.
Race
There is no FDA guidance on the use of Nedosiran with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Nedosiran in patients with renal impairment.
Hepatic Impairment
- No dose adjustment of RIVFLOZA is recommended for patients with mild hepatic impairment (total bilirubin ≤ upper limit of normal [ULN] and aspartate aminotransferase [AST] > ULN or total bilirubin > 1 to 1.5 times ULN and any AST.)
- has not been studied in patients with moderate or severe hepatic impairment (total bilirubin > 1.5 ULN with any AST)
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Nedosiran in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Nedosiran in patients who are immunocompromised.
Administration and Monitoring
Administration
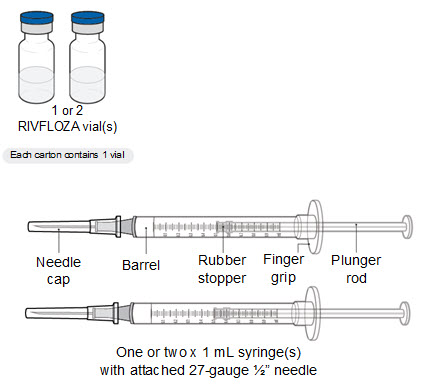
RIVFLOZA Injection 160 mg/mL (present as 170 mg nedosiran sodium salt) is a clear, colorless-to-yellow solution available as follows:
- 80 mg (0.5 mL) single-dose vial
- 128 mg (0.8 mL) single-dose Pre-filled Syringe
- 160 mg (1 mL) single-dose Pre-filled Syringe
Monitoring
- RIVFLOZA can be administered via pre-filled syringe by individuals 12 years and older, and in pediatric patients aged 9 to 11 weighing at least 50 kg, under healthcare professional supervision.
- RIVFLOZA vials should be used under healthcare professional guidance, but caregivers can administer it to pediatric patients after proper training.
Subcutaneous injection into the abdomen or upper thigh is recommended, avoiding veins, scarred, or bruised skin.
- Visual inspection for particulate matter and discoloration before injection is crucial, and cloudy or particle-containing solutions should not be used.
- Dosage administration instructions are provided in the enclosed leaflets.
IV Compatibility
There is limited information regarding the compatibility of Nedosiran and IV administrations.
Overdosage
There is limited information regarding Nedosiran overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Nedosiran Pharmacology in the drug label.
Mechanism of Action
Nedosiran is a double-stranded siRNA (small interfering RNA) that is conjugated to GalNAc (N-acetylgalactosamine) aminosugar residues. Upon subcutaneous administration, the GalNAc-conjugated sugars bind to asialoglycoprotein receptors (ASGPR) present on hepatocytes, facilitating the delivery of nedosiran to these cells. Within hepatocytes, nedosiran acts by reducing levels of hepatic lactate dehydrogenase (LDH) through the degradation of LDHA messenger ribonucleic acid (mRNA) via RNA interference. This mechanism ultimately leads to a reduction in hepatic LDH levels, which in turn decreases the production of oxalate by the liver. Consequently, nedosiran helps alleviate the subsequent burden of oxalate.
Structure
RIVFLOZA injection contains nedosiran, a double-stranded small interfering RNA (siRNA) with four covalently attached N-acetyl-D-galactosamine (GalNAc) residues. Nedosiran targets lactate dehydrogenase A (LDHA) in hepatocytes via GalNAc-mediated delivery.
The structural formula of the nedosiran sodium drug substance is presented below:
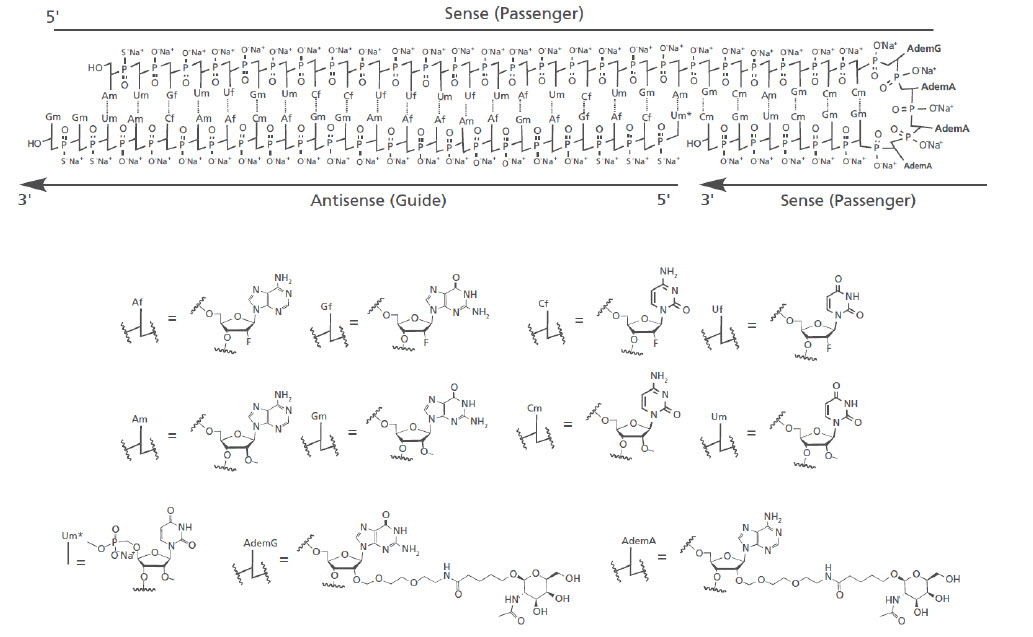
The molecular formula of nedosiran sodium is C662H808F19N231O413P57S6Na57 with a molecular weight of 22,238 Da. Nedosiran sodium is freely soluble in water.
Pharmacodynamics
The pharmacodynamic effects of RIVFLOZA were evaluated after single-dose and monthly-dose administration in patients with PH1. Dose-dependent reductions in urinary oxalate were observed in the single-dose range of 1.5 mg/kg to 6.0 mg/kg. With the recommended monthly dose regimen of RIVFLOZA, onset of effect was observed at the first measurement (30 days after the first dose) and the effect persisted with continued monthly dosing.
Pharmacokinetics
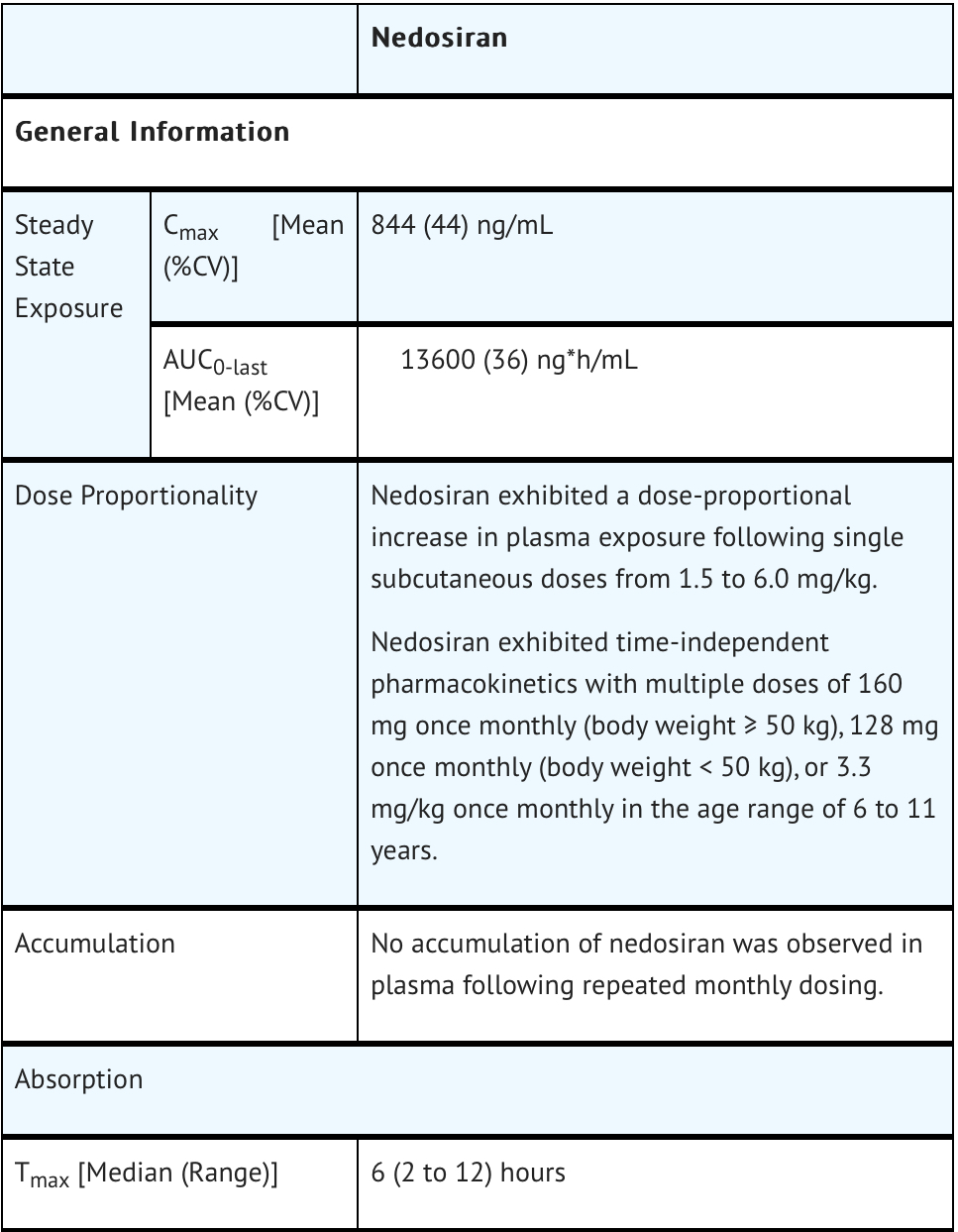
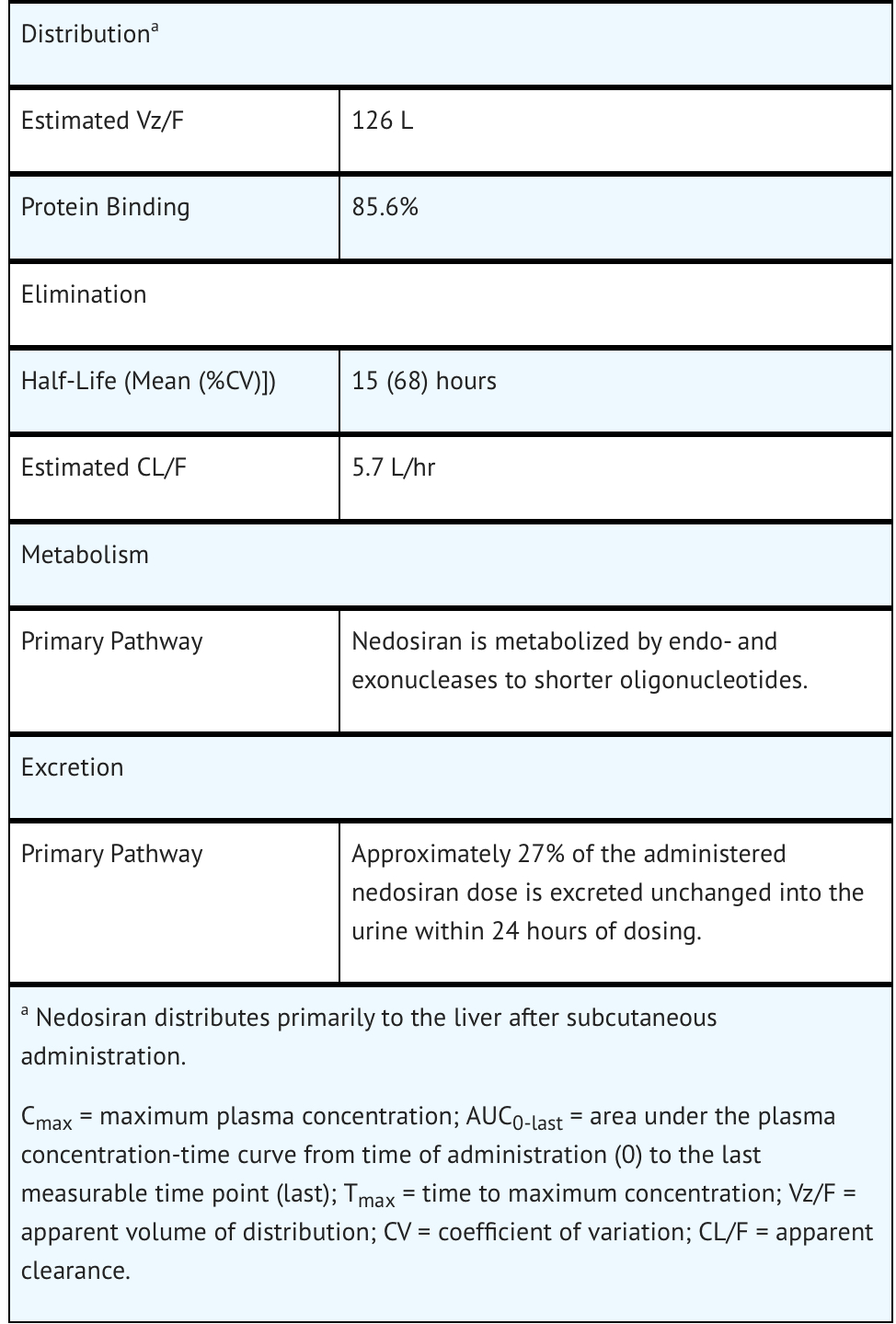
Nonclinical Toxicology
- Carcinogenicity:
Long-term studies assessing the carcinogenic risk of nedosiran have not been conducted.
- Genotoxicity:
Nedosiran did not demonstrate genotoxic effects in bacterial mutagenicity tests, in vitro micronucleus assays using human peripheral blood lymphocytes, or in an in vivo bone marrow micronucleus assay in mice.
- Fertility:
Weekly subcutaneous administration of nedosiran at various doses to male and female mice prior to and throughout mating, as well as during early embryonic development, did not affect fertility or early embryonic development in either males or females.
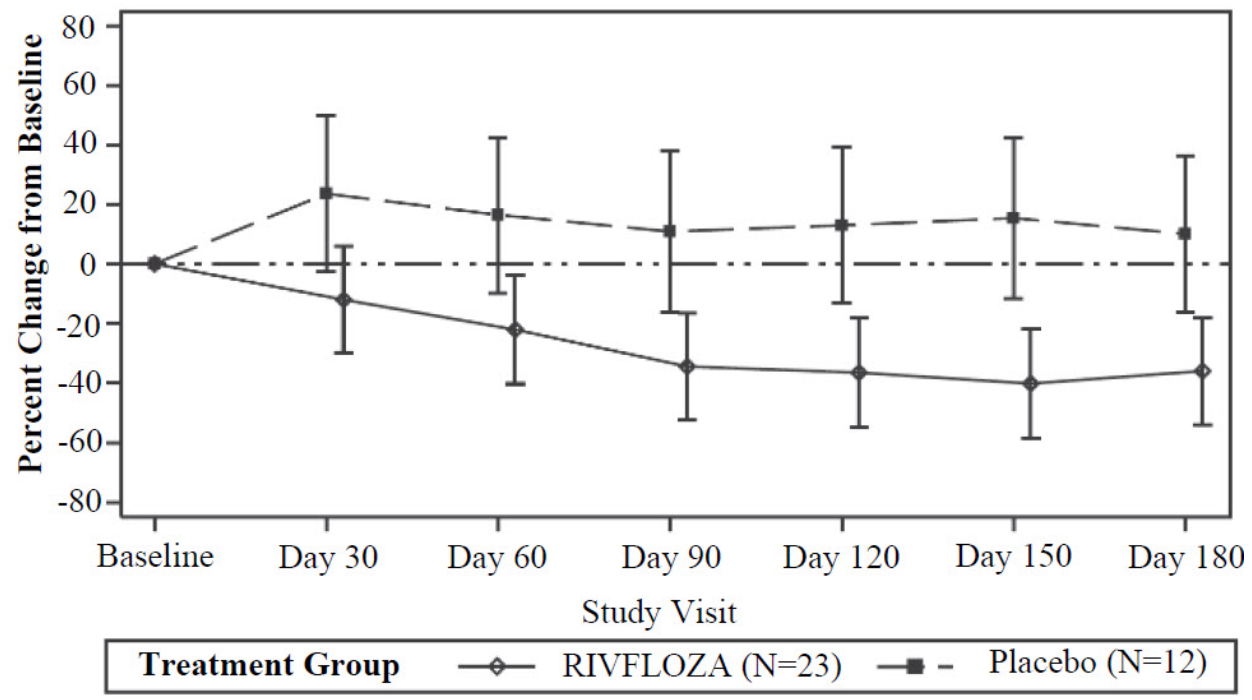
Clinical Studies
PHYOX2 was a randomized, double-blind trial comparing RIVFLOZA and placebo in patients aged 6 years or older with PH1 or PH2 and an eGFR ≥ 30 mL/min/1.73 m2 (NCT03847909). Too few PH2 patients were enrolled to evaluate efficacy in the PH2 population. Therefore, RIVFLOZA is only indicated for patients with PH1. Patients received monthly doses of RIVFLOZA (N=23) or placebo (N=12). The RIVFLOZA dose for patients at least 12 years of age weighing at least 50 kg was 160 mg, for patients at least 12 years of age weighing less than 50 kg was 128 mg, and for children 6 to 11 years of age was 3.3 mg/kg (to a maximum of 128 mg).
How Supplied
Storage
Store refrigerated at 2°C to 8°C (36°F to 46°F). RIVFLOZA can be stored, if needed, at 15°C to 30°C (59°F to 86°F) for a maximum of 28 days (4 weeks). Do not freeze. Store in original carton, away from direct heat and light.
Images
Drug Images
{{#ask: Page Name::Nedosiran |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
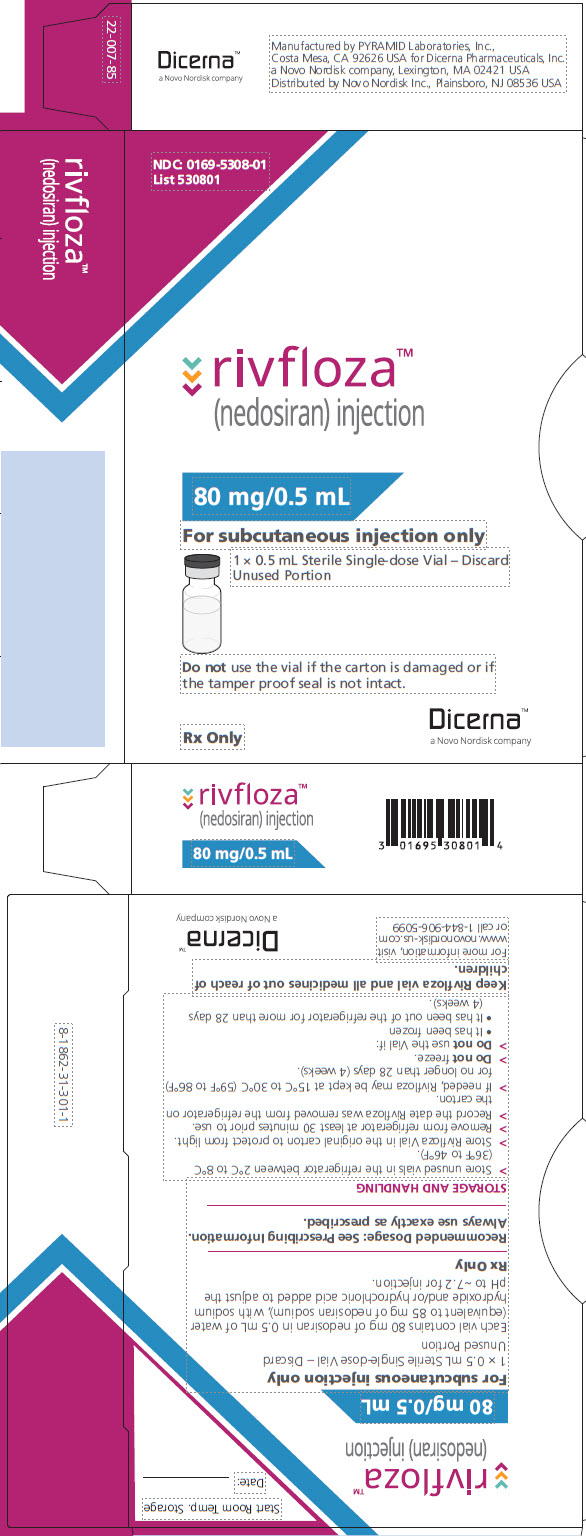

{{#ask: Label Page::Nedosiran |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Indication and Usage: RIVFLOZA is prescribed to lower urinary oxalate levels in individuals with primary hyperoxaluria type 1 (PH1) and relatively preserved kidney function, starting from 9 years of age. Safety Considerations:
Safety and efficacy in children under 9 years old are not established. Inform healthcare provider if pregnant, breastfeeding, or taking any medications, including over-the-counter products and supplements. Administration: Follow Instructions for Use provided with RIVFLOZA carefully. Administer via subcutaneous injection once monthly, with dosage determined by healthcare provider based on body weight. Available as a Pre-filled Syringe or single-dose vial. Healthcare provider or caregiver should administer Pre-filled Syringe to children aged 9 to 11 weighing 110 pounds (50 kilograms) or more. Missed Dose:
- If a dose is missed, administer it as soon as possible. If over 7 days late, inject and resume monthly dosing from the last dose.
Side Effects:
- Common side effects include injection site reactions such as reddening, pain, bruising, rash, or dimpling.
Storage:
- Keep RIVFLOZA refrigerated between 36°F to 46°F (2°C to 8°C).
Can be stored at room temperature (59°F to 86°F or 15°C to 30°C) for up to 28 days. Record removal date from refrigerator on the carton. Do not freeze. Store in original carton away from direct heat and light. Keep out of reach of children. General Information:
- Use RIVFLOZA only as prescribed. Do not share with others or use for conditions not indicated without healthcare provider's approval.

Precautions with Alcohol
Alcohol-Nedosiran interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Nedosiran Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Nedosiran Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.