Levofloxacin labels and packages
Template:Levofloxacin Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Abdurahman Khalil, M.D. [2]
Active Ingredient Made in Japan
Finished Product Manufactured by:
Janssen Ortho LLC, Gurabo, Puerto Rico 00778 (Tablets).
Janssen Pharmaceutica N.V., Beerse, Belgium (Oral Solution, Injection Single-Use Vials).
Hospira, Inc., Austin, TX 78728 (Injection Premix).
Manufactured for:
Janssen Pharmaceuticals, Inc., Titusville, NJ 08560.
© Janssen Pharmaceuticals, Inc.
Revised September 2013
PRINCIPAL DISPLAY PANEL - 250 mg Tablet Carton
10 blister strips 10 tablets each
NDC 50458-920-10
Once-a-day Lēvaquin® tablets (levofloxacin tablets)
Attention Pharmacist: Dispense the accompanying Medication Guide to each patient.
250 mg
Store at 15° to 30°C (59° to 86°F).
Rx only
Package Not Child Resistant
PRINCIPAL DISPLAY PANEL - 500 mg Tablet Bottle Label
50 Tablets
NDC 50458-925-50
Once-a-day Lēvaquin® tablets (levofloxacin tablets)
500mg
Attention Pharmacist: Dispense the accompanying Medication Guide to each patient.
Active Ingredient Made in Japan
Finished Product Manufactured by: JOLLC Gurabo, Puerto Rico 00778
Manufactured for: Janssen Pharmaceuticals, Inc. Titusville, NJ 08560
 |
PRINCIPAL DISPLAY PANEL - 750 mg Tablet Carton
10 blister strips 10 tablets each
NDC 50458-930-10
Once-a-day Lēvaquin® tablets (levofloxacin tablets)
Attention Pharmacist: Dispense the accompanying Medication Guide to each patient.
750 mg
Store at 15° to 30°C (59° to 86°F).
Rx only
Package Not Child Resistant
 |
PRINCIPAL DISPLAY PANEL - 25 mg/mL Oral Solution Carton
NDC 50458-170-01 480 mL
ONCE - A - DAY Lēvaquin® (levofloxacin) Oral Solution
25 mg/mL
Each 1 mL contains 25 mg of levofloxacin in an aqueous solution.
Attention Pharmacist: Dispense the accom- panying Medication Guide to each patient.
Dosage: For information concerning use of LEVAQUIN (levofloxacin) Oral Solution, please see Package Insert.
Bulk package not for retail dispensing.
Dispense in tight, light-resistant container as defined in the USP.
Store bottle at 25°C (77°F); excursions permitted to 15° - 30°C (59° - 86°F)
Keep out of reach of children.
Active Ingredient Made in Japan
Finished Product Manufactured by: Janssen Pharmaceutica, N.V. Beerse, Belgium
Manufactured for: Janssen Pharmaceuticals, Inc. Titusville, NJ 08560
Janssen
Rx only
 |
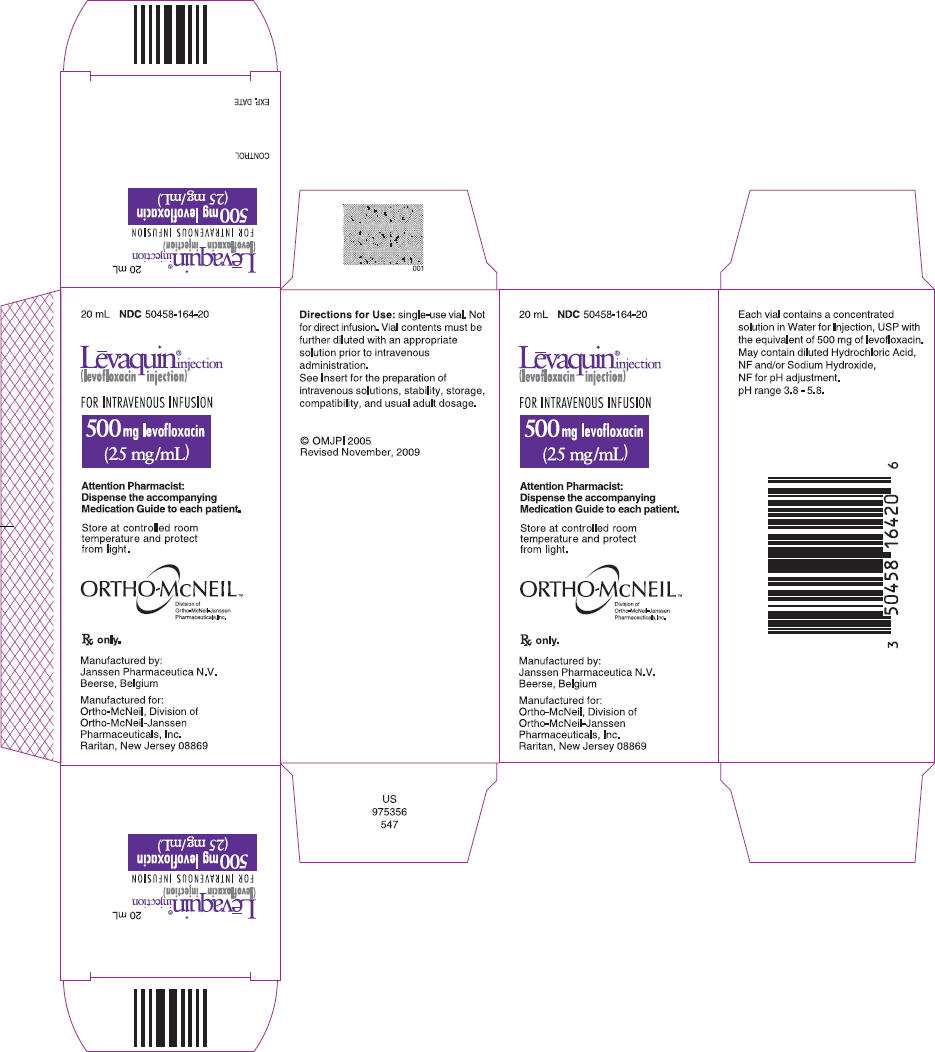
PRINCIPAL DISPLAY PANEL - 500 mg Injection Carton
20 mL NDC 50458-164-20
Lēvaquin® injection (levofloxacin injection)
FOR INTRAVENOUS INFUSION
500 mg levofloxacin (25 mg/mL)
Attention Pharmacist: Dispense the accompanying Medication Guide to each patient.
Store at controlled room temperature and protect from light.
ORTHO-McNEIL™ Division of Ortho-McNeil-Janssen Pharmaceuticals, Inc.
Rx only.
Manufactured by: Janssen Pharmaceutica N.V. Beerse, Belgium
Manufactured for: Ortho-McNeil, Division of Ortho-McNeil-Janssen Pharmaceuticals, Inc. Raritan, New Jersey 08869
 |
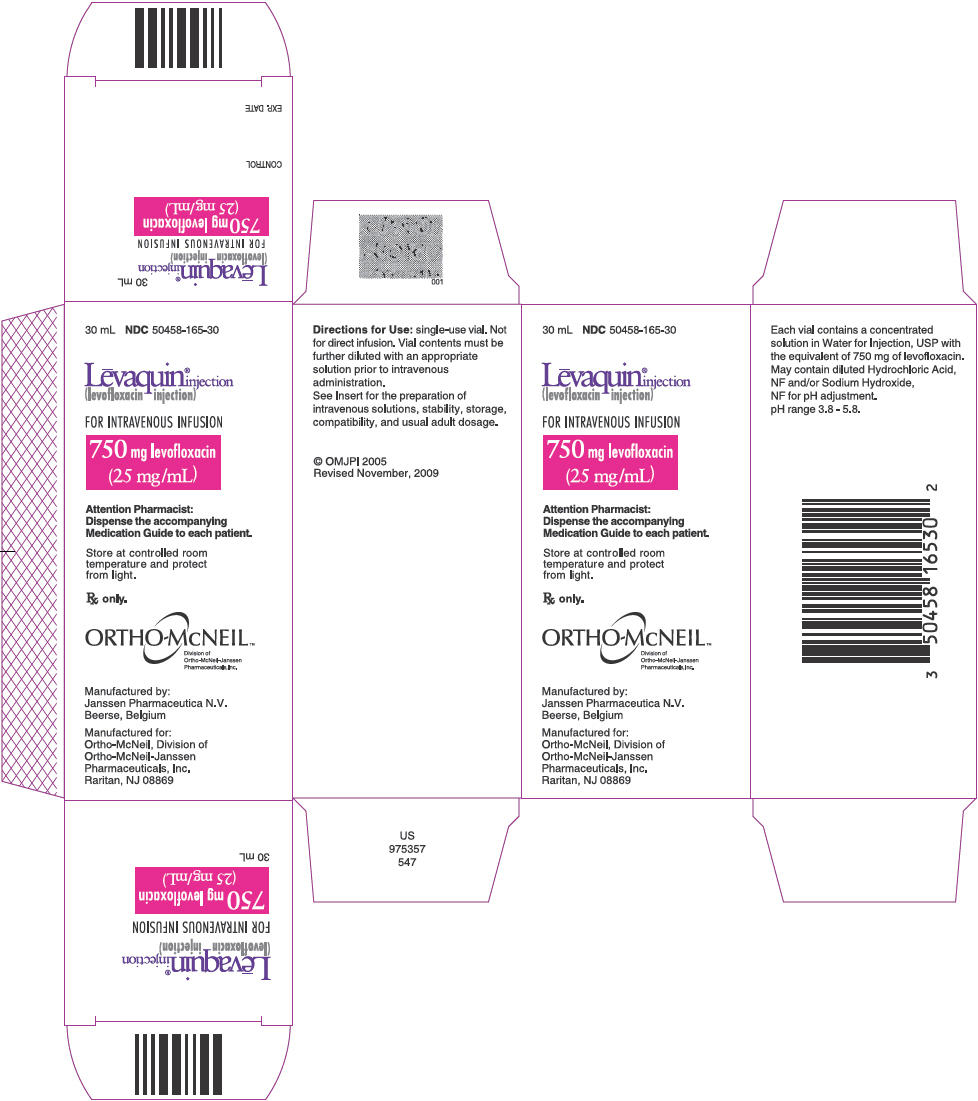
PRINCIPAL DISPLAY PANEL - 750 mg Injection Carton
30 mL NDC 50458-165-30
Lēvaquin® injection (levofloxacin injection)
FOR INTRAVENOUS INFUSION
750 mg levofloxacin (25 mg/mL)
Attention Pharmacist: Dispense the accompanying Medication Guide to each patient.
Store at controlled room temperature and protect from light.
Rx only.
ORTHO-McNEIL™ Division of Ortho-McNeil-Janssen Pharmaceuticals, Inc.
Manufactured by: Janssen Pharmaceutica N.V. Beerse, Belgium
Manufactured for: Ortho-McNeil, Division of Ortho-McNeil-Janssen Pharmaceuticals, Inc. Raritan, NJ 08869
 |
PRINCIPAL DISPLAY PANEL - 5 mg/mL Injection 50 mL Bag
NDC 50458-167-01 DIN 02236839
TO OPEN - TEAR AT NOTCH
One Unit
50 mL
LEVAQUIN® (levofloxacin in 5% dextrose) Injection For Intravenous Infusion 250 mg levofloxacin (5 mg/mL) INFUSE OVER 60 MINUTES
LEAVE BAG IN OVERWRAP UNTIL USE.
Attention Pharmacist: Dispense the accompanying Medication Guide to each patient.
Each 50 mL contains a dilute solution equivalent of 250 mg of levofloxacin (5 mg/mL) in 5% dextrose. May contain Hydrochloric Acid, NF and/or Sodium Hydroxide, NF for pH adjustment. pH range 3.8 - 5.8.
Usual Adult Dosage: See Insert. Recommended storage: At or below 25°C (77°F); however, brief exposure up to 40°C (104°F) does not adversely affect the product. Protect from light. Avoid excessive heat and protect from freezing.
Single-use container. Any unused portion should be discarded. Must not be used in series connection. Additives should not be added or infused through the same intravenous line. The overwrap is a moisture barrier. Do not remove unit from overwrap until ready to use. Use unit promptly when pouch is open. After removing the overwrap, check for minute leaks by squeezing container firmly. If leaks are found, discard unit as sterility may be impaired. Use only if solution is clear and the container is undamaged.
Rx only
Active Ingredient Made in Japan
Finished Product Manufactured by: Hospira, Inc., Austin, TX 78728 Manufactured for: Janssen Pharmaceuticals, Inc. Titusville, NJ 08560 © Janssen Pharmaceuticals, Inc. 2011
10226200 Revised June 2011 WR-0406
 |
PRINCIPAL DISPLAY PANEL - 5 mg/mL Injection 100 mL Bag
Pr
100 mL
NDC 50458-168-01 DIN 02236839
LEVAQUIN® (levofloxacin in 5% dextrose) Injection FOR INTRAVENOUS INFUSION • 500 mg levofloxacin (5 mg/mL) For the US: Attention Pharmacist: Dispense the accompanying Medication Guide to each patient. For Canada: Pharmacist: Dispense with consumer information.
USE IMMEDIATELY ONCE REMOVED FROM THE OVERWRAP. INFUSE OVER 60 MINUTES. No further dilution is necessary. Each 100 mL contains a dilute solution equivalent of 500 mg of levofloxacin (5 mg/mL) in 5% dextrose. May contain Hydrochloric Acid, NF and/or Sodium Hydroxide, NF to adjust pH to 3.8–5.8. Additives should not be added or infused simultaneously through the same intrave- nous line. Single-use container. Any unused portion should be discarded. Usual Adult Dosage: See Insert. Sterile (stérile), nonpyrogenic. Use only if solution is clear and container is undamaged. Must not be used in series connection.
Active Ingredient Made in Japan
Finished Product Manufactured by: Hospira, Inc., Austin, TX 78728 Manufactured for: Janssen Pharmaceuticals,Inc. Titusville, NJ 08560 and Janssen Inc., Toronto, Canada M3C 1L9
Rx only IM-2139 Rev. 6/11
500 mg
© Janssen 2011 10226500
 |
PRINCIPAL DISPLAY PANEL - 5 mg/mL Injection 150 mL Bag Label
TO OPEN - TEAR AT NOTCH
NDC 50458-166-01 DIN 02236839
One Unit
150 mL
LEVAQUIN® (levofloxacin in 5% dextrose) Injection For Intravenous Infusion
750 mg levofloxacin (5 mg/mL)
INFUSE OVER 90 MINUTES
LEAVE BAG IN OVERWRAP UNTIL USE.
Attention Pharmacist: Dispense the accompanying Medication Guide to each patient.
Each 150 mL contains a dilute solution equivalent of 750 mg of levofloxacin (5 mg/mL) in 5% dextrose. May contain Hydrochloric Acid, NF and/or Sodium Hydroxide, NF for pH adjustment. pH range 3.8 - 5.8.
Usual Adult Dosage: See Insert. Recommended storage: At or below 25°C (77°F); however, brief exposure up to 40°C (104°F) does not adversely affect the product. Protect from light. Avoid excessive heat and protect from freezing.
Single-use container. Any unused portion should be discarded. Must not be used in series connection. Additives should not be added or infused through the same intravenous line. The overwrap is a moisture barrier. Do not remove unit from overwrap until ready to use. Use unit promptly when pouch is open. After removing the overwrap, check for minute leaks by squeezing container firmly. If leaks are found, discard unit as sterility may be impaired. Use only if solution is clear and the container is undamaged.
Rx only
Active Ingredient Made in Japan
Finished Product Manufactured by: Hospira, Inc., Austin, TX 78728 Manufactured for: Janssen Pharmaceuticals, Inc. Titusville, NJ 08560 © Janssen Pharmaceuticals, Inc. 2011
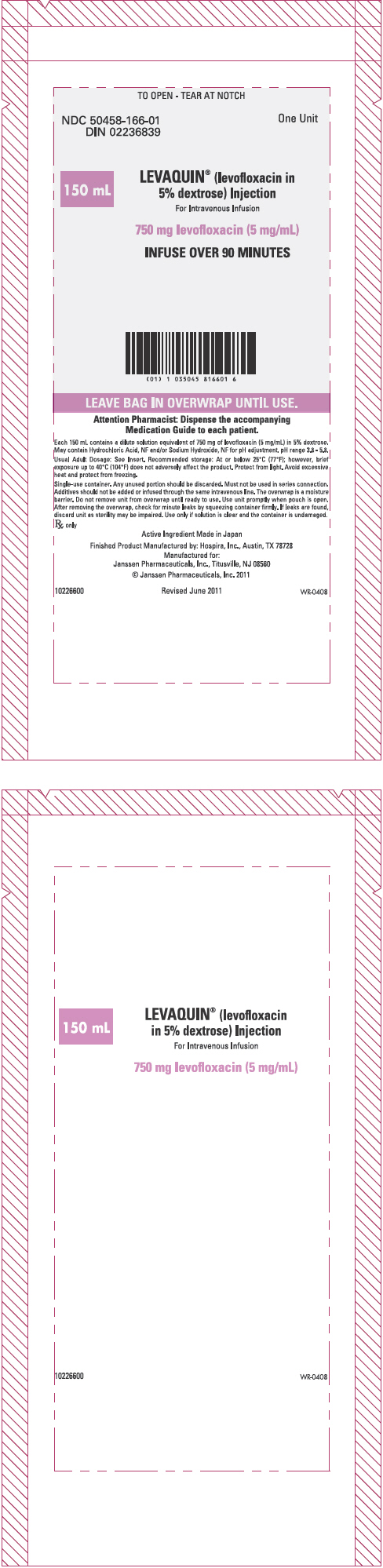 |
10226600 Revised June 2011 WR-0408