Levobunolol hydrochloride
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Levobunolol hydrochloride is a beta blocker that is FDA approved for the treatment of chronic open-angle glaucoma or ocular hypertension.. Common adverse reactions include ocular burning and stinging, headache, asthenia, chest pain, bradycardia, arrhythmia, hypotension, syncope, nausea, diarrhea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Levobunolol Hydrochloride Ophthalmic Solution has been shown to be effective in lowering intraocular pressure and may be used in patients with chronic open-angle glaucoma or ocular hypertension.
Dosage
- The recommended starting dose is one to two drops of Levobunolol hydrochloride Ophthalmic Solution 0.5% in the affected eye(s) once a day. In patients with more severe or uncontrolled glaucoma, Levobunolol Hydrochloride Ophthalmic Solution 0.5% can be administered b.i.d. As with any new medication, careful monitoring of patients is advised.
- Dosages above one drop of Levobunolol hydrochloride Ophthalmic Solution 0.5% b.i.d. are not generally more effective. If the patient's IOP is not at a satisfactory level on this regimen, concomitant therapy with dipivefrin and/or epinephrine, and/or pilocarpine and other miotics, and/or systemically administered carbonic anhydrase inhibitors, such as acetazolamide, can be instituted. Patients should not typically use two or more topical ophthalmic beta-adrenergic blocking agents simultaneously.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Levobunolol hydrochloride in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Levobunolol hydrochloride in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Levobunolol hydrochloride in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Levobunolol hydrochloride in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Levobunolol hydrochloride in pediatric patients.
Contraindications
- Levobunolol hydrochloride Ophthalmic Solution is contraindicated in those individuals with bronchial asthma or with a history of bronchial asthma, or severe chronic obstructive pulmonary disease ; sinus bradycardia, second and third degree atrioventricular block; overt cardiac failure; cardiogenic shock; or hypersensitivity to any component of these products.
Warnings
- As with other topically applied ophthalmic drugs, Levobunolol hydrochloride Ophthalmic Solution may be absorbed systemically. The same adverse reactions found with systemic administration of beta-adrenergic blocking agents may occur with topical administration. For example, severe respiratory reactions and cardiac reactions, including death due to bronchospasm in patients with asthma, and rarely death in association with cardiac failure, have been reported with topical application of beta-adrenergic blocking agents.
Cardiac Failure:
- Sympathetic stimulation may be essential for support of the circulation in individuals with diminished myocardial contractility, and its inhibition by beta-adrenergic receptor blockade may precipitate more severe failure.
- In Patients Without a History of Cardiac Failure: Continued depression of the myocardium with beta-blocking agents over a period of time can, in some cases, lead to cardiac failure. At the first sign or symptom of cardiac failure, Levobunolol hydrochloride Ophthalmic Solution should be discontinued.
Obstructive Pulmonary Disease:
- PATIENTS WITH CHRONIC OBSTRUCTIVE PULMONARY DISEASE (e.g., CHRONIC BRONCHITIS, EMPHYSEMA) OF MILD OR MODERATE SEVERITY, BRONCHOSPASTIC DISEASE OR A HISTORY OF BRONCHOSPASTIC DISEASE (OTHER THAN BRONCHIAL ASTHMA OR A HISTORY OF BRONCHIAL ASTHMA, IN WHICH LEVOBUNOLOL HYDROCHLORIDE OPHTHALMIC SOLUTION IS CONTRAINDICATED,SHOULD IN GENERAL NOT RECEIVE BETA BLOCKERS, INCLUDING Levobunolol Hydrochloride Ophthalmic Solution. However, if Levobunolol hydrochloride Ophthalmic Solution is deemed necessary in such patients, then it should be administered cautiously since it may block bronchodilation produced by endogenous and exogenous catecholamine stimulation of beta2 receptors.
Major Surgery:
- The necessity or desirability of withdrawal of beta-adrenergic blocking agents prior to major surgery is controversial. Beta-adrenergic receptor blockade impairs the ability of the heart to respond to beta-adrenergically mediated reflex stimuli. This may augment the risk of general anesthesia in surgical procedures. Some patients receiving beta-adrenergic receptor blocking agents have been subject to protracted severe hypotension during anesthesia. Difficulty in restarting and maintaining the heartbeat has also been reported. For these reasons, in patients undergoing elective surgery, gradual withdrawal of beta-adrenergic receptor blocking agents may be appropriate.
- If necessary during surgery, the effects of beta-adrenergic blocking agents may be reversed by sufficient doses of such agonists as isoproterenol, dopamine, dobutamine or levarterenol.
Diabetes Mellitus:
- Beta-adrenergic blocking agents should be administered with caution in patients subject to spontaneous hypoglycemia or to diabetic patients (especially those with labile diabetes) who are receiving insulin or oral hypoglycemic agents. Beta-adrenergic receptor blocking agents may mask the signs and symptoms of acute hypoglycemia.
Thyrotoxicosis:
- Beta-adrenergic blocking agents may mask certain clinical signs (e.g., tachycardia) of hyperthyroidism. Patients suspected of developing thyrotoxicosis should be managed carefully to avoid abrupt withdrawal of beta-adrenergic blocking agents which might precipitate a thyroid storm.
- These products contain sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in non asthmatic people.
Adverse Reactions
Clinical Trials Experience
- In clinical trials, the use of Levobunolol hydrochloride Ophthalmic Solution has been associated with transient ocular burning and stinging in up to 1 in 3 patients, and with blepharoconjunctivitis in up to 1 in 20 patients. Decreases in heart rate and blood pressure have been reported.
- The following adverse effects have been reported rarely with the use of Levobunolol Hydrochloride Ophthalmic Solution: iridocyclitis, headache, transient ataxia, dizziness, lethargy, urticaria and pruritus. Decreased corneal sensitivity has been noted in a small number of patients. Although levobunolol has minimal membrane-stabilizing activity, there remains a possibility of decreased corneal sensitivity after prolonged use.
- The following additional adverse reactions have been reported either with Levobunolol Hydrochloride Ophthalmic Solution or ophthalmic use of other beta-adrenergic receptor blocking agents:
- BODY AS A WHOLE: Headache, asthenia, chest pain.
- CARDIOVASCULAR: Bradycardia, arrhythmia, hypotension, syncope, heart block, cerebral vascular accident, cerebral ischemia, congestive heart failure, palpitation, cardiac arrest.
- PSYCHIATRIC: Depression, confusion, increase in signs and symptoms of myasthenia gravis, paresthesia.
- SKIN: Hypersensitivity, including localized and generalized rash, alopecia, Stevens-Johnson Syndrome.
- RESPIRATORY: Bronchospasm (predominantly in patients with pre-existing bronchospastic disease), respiratory failure, dyspnea, nasal congestion.
- UROGENITAL: Impotence.
- ENDOCRINE: Masked symptoms of hypoglycemia in insulin-dependent diabetics.
- Other reactions associated with the oral use of non-selective adrenergic receptor blocking agents should be considered potential effects with ophthalmic use of these agents.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Levobunolol hydrochloride in the drug label.
Drug Interactions
- Although Levobunolol hydrochloride Ophthalmic Solution used alone has little or no effect on pupil size, mydriasis resulting from concomitant therapy with Levobunolol Hydrochloride Ophthalmic Solution and epinephrine may occur.
- Close observation of the patient is recommended when a beta-blocker is administered to patients receiving catecholamine-depleting drugs such as reserpine, because of possible additive effects and the production of hypotension and/or marked bradycardia, which may produce vertigo, syncope, or postural hypotension. Patients receiving beta-adrenergic blocking agents along with either oral or intravenous calcium antagonists should be monitored for possible atrioventricular conduction disturbances, left ventricular failure and hypotension. In patients with impaired cardiac function, simultaneous use should be avoided altogether. The concomitant use of beta-adrenergic blocking agents with digitalis and calcium antagonists may have additive effects on prolonging atrioventricular conduction time.
- Phenothiazine-related compounds and beta-adrenergic blocking agents may have additive hypotensive effects due to the inhibition of each other's metabolism.
Use in Specific Populations
Pregnancy
- Pregnancy Category C. Fetotoxicity (as evidenced by a greater number of resorption sites) has been observed in rabbits when doses of Levobunolol HCl equivalent to 200 and 700 times the recommended dose for the treatment of glaucoma were given. No fetotoxic effects have been observed in similar studies with rats at up to 1,800 times the human dose for glaucoma. Teratogenic studies with Levobunolol in rats at doses up to 25 mg/kg/day (1,800 times the recommended human dose for glaucoma) showed no evidence of fetal malformations. There were no adverse effects on postnatal development of offspring. It appears when results from studies using rats and studies with other beta-adrenergic blockers are examined, that the rabbit may be a particularly sensitive species. There are no adequate and well controlled studies in pregnant women. Levobunolol hydrochloride Ophthalmic Solution should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Levobunolol hydrochloride in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Levobunolol hydrochloride during labor and delivery.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Systemic beta-blockers and topical timolol maleate are known to be excreted in human milk. Caution should be exercised when Levobunolol hydrochloride Ophthalmic Solution is administered to a nursing woman.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
- No overall differences in safety or effectiveness have been observed between elderly and younger patients.
Gender
There is no FDA guidance on the use of Levobunolol hydrochloride with respect to specific gender populations.
Race
There is no FDA guidance on the use of Levobunolol hydrochloride with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Levobunolol hydrochloride in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Levobunolol hydrochloride in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Levobunolol hydrochloride in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Levobunolol hydrochloride in patients who are immunocompromised.
Administration and Monitoring
Administration
- Topical
Monitoring
There is limited information regarding Monitoring of Levobunolol hydrochloride in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Levobunolol hydrochloride in the drug label.
Overdosage
- No data are available regarding overdosage in humans. Should accidental ocular overdosage occur, flush eye(s) with water or normal saline. If accidentally ingested, efforts to decrease further absorption may be appropriate (gastric lavage). The most common signs and symptoms to be expected with overdosage with administration of a systemic beta-adrenergic blocking agent are symptomatic bradycardia, hypotension, bronchospasm, and acute cardiac failure. Should these symptoms occur, discontinue Levobunolol hydrochloride Ophthalmic Solution therapy and initiate appropriate supportive therapy. The following supportive measures should be considered:
- 1. Symptomatic bradycardia: Use atropine sulfate intravenously in a dosage of 0.25 mg to 2 mg to induce vagal blockade. If bradycardia persists, intravenous isoproterenol hydrochloride should be administered cautiously. In refractory cases, the use of a transvenous cardiac pacemaker should be considered.
- 2. Hypotension: Use sympathomimetic pressor drug therapy such as dopamine, dobutamine or levarterenol. In refractory cases, the use of glucagon hydrochloride may be useful.
- 3. Bronchospasm: Use isoproterenol hydrochloride. Additional therapy with aminophylline may be considered.
- 4. Acute cardiac failure: Conventional therapy with digitalis, diuretics and oxygen should be instituted immediately. In refractory cases, the use of intravenous aminophylline is suggested. This may be followed, if necessary, by glucagon hydrochloride which may be useful.
- 5. Heart block (second or third degree): Use isoproterenol hydrochloride or a transvenous cardiac pacemaker.
Pharmacology
Mechanism of Action
There is limited information regarding Levobunolol hydrochloride Mechanism of Action in the drug label.
Structure
- Levobunolol Hydrochloride Ophthalmic Solution is a sterile, noncardioselective beta-adrenoceptor blocking agent for ophthalmic use. The solution is colorless to slightly light yellow in appearance with an osmolality range of 250-360 mOsm/kg. The shelf life pH range is 5.5 to 7.5.
Structural Formula: levobunolol HCl
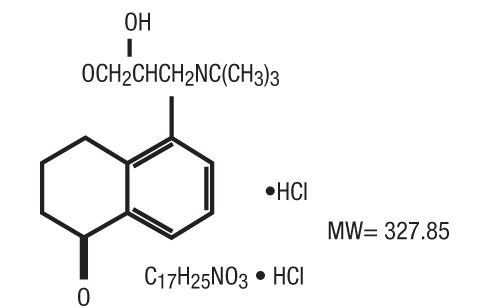
Chemical Name: (-)-5-[3-(tert-Butylamino)-2-hydroxypropoxy]-3,4-dihydro-1(2H)-naphthalenone hydrochloride.
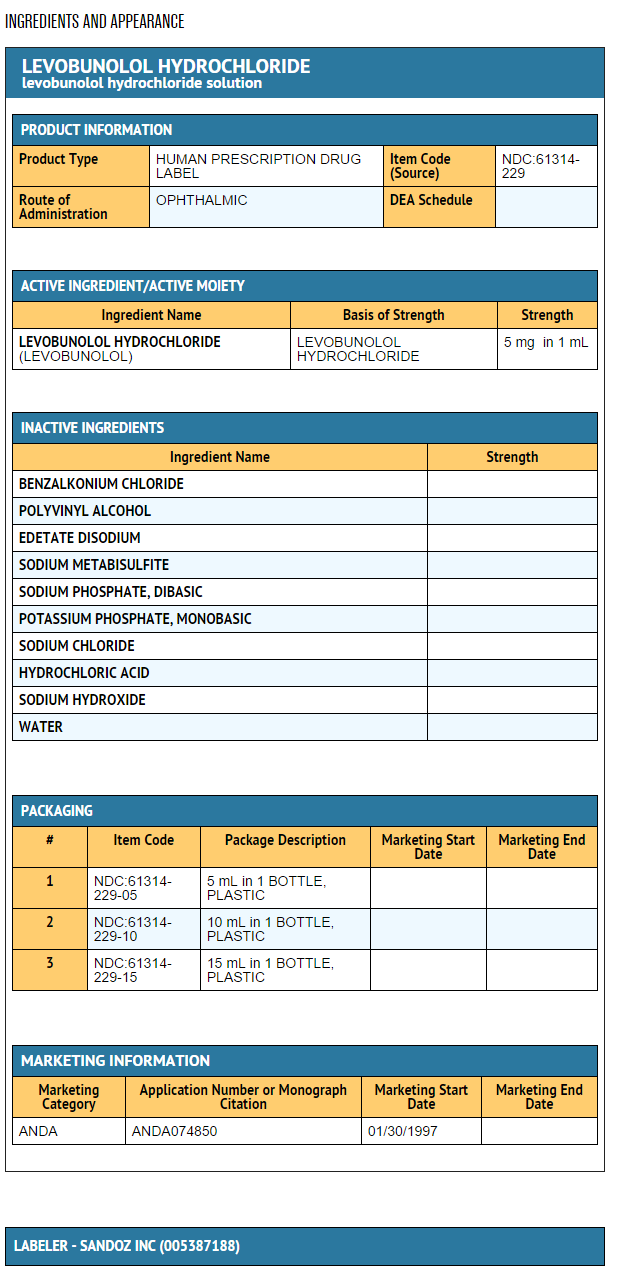
Each mL of 0.5% contains:
Active: levobunolol HCl 0.5%. Preservative: benzalkonium chloride 0.004%. Inactives: polyvinyl alcohol 1.4%; edetate disodium; sodium metabisulfite; sodium phosphate, dibasic; potassium phosphate, monobasic; sodium chloride; hydrochloric acid or sodium hydroxide to adjust pH; and purified water.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Levobunolol hydrochloride in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Levobunolol hydrochloride in the drug label.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- In a lifetime oral study in mice, there were statistically significant (p ≤ 0.05) increases in the incidence of benign leiomyomas in female mice at 200 mg/kg/day (14,000 times the recommended human dose for glaucoma), but not at 12 or 50 mg/kg/day (850 and 3,500 times the human dose). In a two-year oral study of levobunolol HCl in rats, there was a statistically significant (p ≤ 0.05) increase in the incidence of benign hepatomas in male rats administered 12,800 times the recommended human dose for glaucoma. Similar differences were not observed in rats administered oral doses equivalent to 350 times to 2,000 times the recommended human dose for glaucoma.
- Levobunolol did not show evidence of mutagenic activity in a battery of microbiological and mammalian in vitro and in vivo assays.
- Reproduction and fertility studies in rats showed no adverse effect on male or female fertility at doses up to 1,800 times the recommended human dose for glaucoma.
Clinical Studies
There is limited information regarding Clinical Studies of Levobunolol hydrochloride in the drug label.
How Supplied
- Levobunolol Hydrochloride Ophthalmic Solution, USP is sterile and supplied in white, opaque, plastic ophthalmic dispensers as follows:
- Levobunolol Hydrochloride Ophthalmic Solution 0.5%:
- 5 mL NDC 61314-229-05
- 10 mL NDC 61314-229-10
- 15 mL NDC 61314-229-15
Storage
- Protect from light. Store at 15° - 30°C (59° - 86°F).
Images
Drug Images
{{#ask: Page Name::Levobunolol hydrochloride |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Levobunolol hydrochloride |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Levobunolol hydrochloride in the drug label.
Precautions with Alcohol
- Alcohol-Levobunolol hydrochloride interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- LEVOBUNOLOL HYDROCHLORIDE
Look-Alike Drug Names
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Levobunolol hydrochloride
|Pill Name=Levobunolol ingredients and appearance.png
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Levobunolol hydrochloride |Label Name=Levobunolol image.jpg
}}
{{#subobject:
|Label Page=Levobunolol hydrochloride |Label Name=
}}