JAK-STAT signaling pathway
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
The JAK-STAT signaling pathway takes part in the regulation of cellular responses to cytokines and growth factors. Employing Janus kinases (JAKs) and Signal Transducers and Activators of Transcription (STATs), the pathway transduces the signal carried by these extracellular polypeptides to the cell nucleus, where activated STAT proteins modify gene expression. Although STATs were originally discovered as targets of Janus kinases, it has now become apparent that certain stimuli can activate them independent of JAKs. The pathway plays a central role in principal cell fate decisions, regulating the processes of cell proliferation, differentiation and apoptosis. It is particularly important in hematopoiesis - production of blood cells.
Mechanism
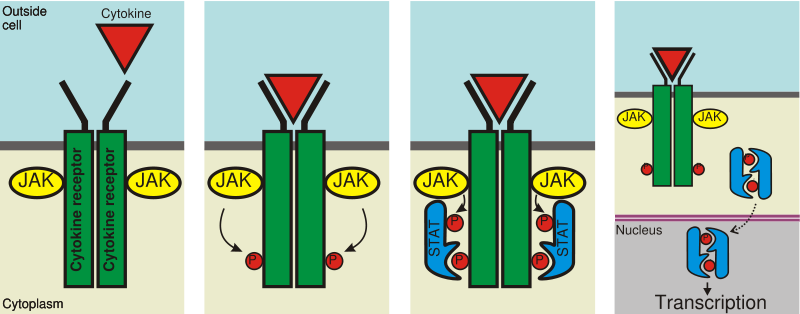
JAKs, which have tyrosine kinase activity, bind to some cell surface cytokine receptors. The binding of the ligand to the receptor triggers activation of JAKs. With increased kinase activity, they phosphorylate tyrosine residues on the receptor and create sites for interaction with proteins that contain phosphotyrosine-binding SH2 domain. STATs possessing SH2 domains capable of binding these phosphotyrosine residues, are recruited to the receptors and are themselves tyrosine-phosphorylated by JAKs. These phosphotyrosines then act as docking sites for SH2 domains of other STATs, mediating their dimerisation. Different STATs form hetero- as well as homodimers. Activated STAT dimers accumulate in the cell nucleus and activate transcription of their target genes.[1] STATs may also be tyrosine-phosphorylated by other non-receptor tyrosine kinases, such as c-src, as well as receptor tyrosine kinases, such as the epidermal growth factor receptor.
The pathway is negatively regulated on multiple levels. Protein tyrosine phosphatases remove phosphates from cytokine receptors as well as activated STATs.[1] More recently identified Suppressors of Cytokine Signaling (SOCS) inhibit STAT phosphorylation by binding and inhibiting JAKs or competing with STATs for phosphotyrosine binding sites on cytokine receptors.[2] STATs are also negatively regulated by Protein Inhibitors of Activated STATs (PIAS), which act in the nucleus through several mechanisms.[3] For example, PIAS1 and PIAS3 inhibit transcriptional activation by STAT1 and STAT3 respectively by binding and blocking access to the DNA sequences they recognise.
References
- ↑ 1.0 1.1 Template:Cite online journal
- ↑ D. L. Krebs and D. J. Hilton. (2001) "SOCS proteins: negative regulators of cytokine signaling" in Stem Cells Volume 19, pages 378-387. Template:Entrez Pubmed
- ↑ Template:Cite online journal
- Schroder, K., P. J. Hertzog, T. Ravasi & D. A. Hume (2004) "Interferon-γ: an overview of signals, mechanisms and functions". Journal of Leukocyte Biology 75:163-189.
- O'Shea, J. J., M. Gadina & R. D. Schreiber (2002) "Cytokine Signaling in 2002: New Surprises in the Jak/Stat Pathway". Cell 109, S121-S131.