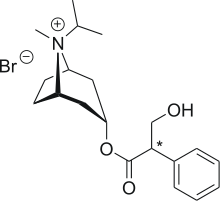
Ipratropium (inhalation)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Adeel Jamil, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Ipratropium (inhalation) is an anticholinergic agent that is FDA approved for the treatment of bronchospasm associated with chronic obstructive pulmonary disease, including chronic bronchitis and emphysema. Common adverse reactions include headache, dizziness, nervousness, back pain, chest pain, mouth dryness, nausea, coughing, dyspnea, bronchitis and upper respiratory tract infections.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Ipratropium Bromide Inhalation Solution administered either alone or with other bronchodilators, especially beta adrenergics, is indicated as a bronchodilator for maintenance treatment of bronchospasm associated with chronic obstructive pulmonary disease, including chronic bronchitis and emphysema.
Dosing Information
- The usual dosage of ipratropium bromide inhalation solution is 500 mcg (1 Unit-Dose Vial) administered three to four times a day by oral nebulization, with doses 6 to 8 hours apart. Ipratropium bromide inhalation solution unit-dose vials contain 500 mcg ipratropium bromide, USP anhydrous in 2.5 mL normal saline. Ipratropium bromide inhalation solution can be mixed in the nebulizer with albuterol or metaproterenol if used within one hour. Drug stability and safety of Ipratropium Bromide Inhalation Solution when mixed with other drugs in a nebulizer have not been established.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ipratropium (inhalation) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ipratropium (inhalation) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Ipratropium (inhalation) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ipratropium (inhalation) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ipratropium (inhalation) in pediatric patients.
Contraindications
- Ipratropium bromide is contraindicated in known or suspected cases of hypersensitivity to ipratropium bromide, or to atropine and its derivatives.
Warnings
- The use of ipratropium bromide inhalation solution as a single agent for the relief of bronchospasm in acute COPD exacerbation has not been adequately studied. Drugs with faster onset of action may be preferable as initial therapy in this situation. Combination of ipratropium bromide and beta agonists has not been shown to be more effective than either drug alone in reversing the bronchospasm associated with acute COPD exacerbation.
- Immediate hypersensitivity reactions may occur after administration of ipratropium bromide, as demonstrated by rare cases of urticaria, angioedema, rash, bronchospasm and oropharyngeal edema.
PRECAUTIONS
General
- Ipratropium bromide should be used with caution in patients with narrow-angle glaucoma, prostatic hypertrophy or bladder-neck obstruction.
Adverse Reactions
Clinical Trials Experience
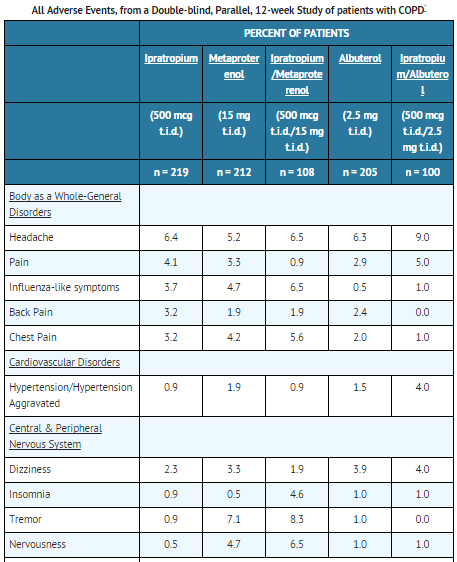
- Adverse reaction information concerning ipratropium bromide inhalation solution is derived from 12-week active-controlled clinical trials. Additional information is derived from foreign post-marketing experience and the published literature.
- All adverse events, regardless of drug relationship, reported by three percent or more patients in the 12-week controlled clinical trials appear in the table below.
- Additional adverse reactions reported in less than three percent of the patients treated with ipratropium bromide include tachycardia, palpitations, eye pain, urinary retention, urinary tract infection and urticaria. Cases of precipitation or worsening of narrow-angle glaucoma, mydriasis, and acute eye pain have been reported.
- Lower respiratory adverse reactions (bronchitis, dyspnea and bronchospasm) were the most common events leading to discontinuation of ipratropium bromide therapy in the 12-week trials. Headache, mouth dryness and aggravation of COPD symptoms are more common when the total daily dose of ipratropium bromide equals or exceeds 2,000 mcg.
- Allergic-type reactions such as skin-rash, angioedema of tongue, lips and face, urticaria, laryngospasm and anaphylactic reaction have been reported. Many of the patients had a history of allergies to other drugs and/or foods.

Postmarketing Experience
There is limited information regarding Ipratropium (inhalation) Postmarketing Experience in the drug label.
Drug Interactions
- Ipratropium bromide has been shown to be a safe and effective bronchodilator when used in conjunction with beta adrenergic bronchodilators. Ipratropium bromide has also been used with other pulmonary medications, including methylxanthines and corticosteroids, without adverse drug interactions.
Use in Specific Populations
Pregnancy
- Oral reproduction studies performed in mice, rats and rabbits at doses of 10, 100, and 125 mg/kg respectively, and inhalation reproduction studies in rats and rabbits at doses of 1.5 and 1.8 mg/kg (or approximately 38 and 45 times the recommended human daily dose) respectively, have demonstrated no evidence of teratogenic effects as a result of ipratropium bromide. However, no adequate or well-controlled studies have been conducted in pregnant women. Because animal reproduction studies are not always predictive of human response, ipratropium bromide should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ipratropium (inhalation) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Ipratropium (inhalation) during labor and delivery.
Nursing Mothers
- It is not known whether ipratropium bromide is excreted in human milk. Although lipid-insoluble quaternary bases pass into breast milk, it is unlikely that ipratropium bromide would reach the infant to a significant extent, especially when taken by inhalation since ipratropium bromide is not well absorbed systemically after inhalation or oral administration. However, because many drugs are excreted in human milk, caution should be exercised when ipratropium bromide is administered to a nursing woman.
Pediatric Use
- Safety and effectiveness in the pediatric population below the age of 12 have not been established.
Geriatic Use
There is no FDA guidance on the use of Ipratropium (inhalation) in geriatric settings.
Gender
There is no FDA guidance on the use of Ipratropium (inhalation) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ipratropium (inhalation) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Ipratropium (inhalation) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Ipratropium (inhalation) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Ipratropium (inhalation) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Ipratropium (inhalation) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Inhalation
Monitoring
There is limited information regarding Ipratropium (inhalation) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Ipratropium (inhalation) and IV administrations.
Overdosage
- Acute systemic overdosage by inhalation solution is unlikely since ipratropium bromide is not well absorbed after inhalation at up to four-fold the recommended dose, or after oral administration at up to forty-fold the recommended dose. The oral LD50 of ipratropium bromide ranged between 1001 and 2010 mg/kg in mice; between 1667 and 4000 mg/kg in rats; and between 400 and 1300 mg/kg in dogs.
Pharmacology
Mechanism of Action
- Ipratropium bromide is an anticholinergic (parasympatholytic) agent that, based on animal studies, appears to inhibit vagally mediated reflexes by antagonizing the action of acetylcholine, the transmitter agent released from the vagus nerve.
- Anticholinergics prevent the increases in intracellular concentration of cyclic guanosine monophosphate (cyclic GMP) that are caused by interaction of acetylcholine with the muscarinic receptor on bronchial smooth muscle.
Structure
- The active ingredient, ipratropium bromide monohydrate, USP, is an anticholinergic bronchodilator chemically described as 8-azoniabicyclo [3.2.1]- octane, 3-(3-hydroxy-1-oxo-2-phenylpropoxy)-8-methyl-8-(1-methylethyl)-, bromide, monohydrate (endo, syn)-, (±)-; a synthetic quaternary ammonium compound, chemically related to atropine.

- Ipratropium Bromide Monohydrate C20H30BrNO3•H2O Mol.Wt. 430.4
- Ipratropium bromide is a white crystalline substance, freely soluble in water and lower alcohols. It is a quaternary ammonium compound and thus exists in an ionized state in aqueous solutions. It is relatively insoluble in non-polar media.
- Ipratropium Bromide Inhalation Solution is administered by oral inhalation with the aid of a nebulizer. It contains ipratropium bromide, USP 0.02% (anhydrous basis) in a sterile, preservative-free, isotonic saline solution, pH-adjusted to 3.4 (3 to 4) with hydrochloric acid.
Pharmacodynamics
There is limited information regarding Ipratropium (inhalation) Pharmacodynamics in the drug label.
Pharmacokinetics
- The bronchodilation following inhalation of ipratropium bromide is primarily a local, site-specific effect, not a systemic one. Much of an administered dose is swallowed but not absorbed, as shown by fecal excretion studies. Following nebulization of a 2 mg dose, a mean 7% of the dose was absorbed into the systemic circulation either from the surface of the lung or from the gastrointestinal tract. The half-life of elimination is about 1.6 hours after intravenous administration. Ipratropium bromide is minimally (0 to 9% in vitro) bound to plasma albumin and a1-acid glycoproteins. It is partially metabolized. Autoradiographic studies in rats have shown that ipratropium bromide does not penetrate the blood-brain barrier. Ipratropium bromide has not been studied in patients with hepatic or renal insufficiency. It should be used with caution in those patient populations.
- In controlled 12-week studies in patients with bronchospasm associated with chronic obstructive pulmonary disease (chronic bronchitis and emphysema) significant improvements in pulmonary function (FEV1 increases of 15% or more) occurred within 15 to 30 minutes, reached a peak in 1 to 2 hours, and persisted for periods of 4 to 5 hours in the majority of patients, with about 25% to 38% of the patients demonstrating increases of 15% or more for at least 7 to 8 hours. Continued effectiveness of ipratropium bromide inhalation solution was demonstrated throughout the 12-week period. In addition, significant increases in forced vital capacity (FVC) have been demonstrated. However, ipratropium bromide did not consistently produce significant improvement in subjective symptom scores nor in quality of life scores over the 12-week duration of study.
- Additional controlled 12-week studies were conducted to evaluate the safety and effectiveness of ipratropium bromide inhalation solution administered concomitantly with the beta adrenergic bronchodilator solutions metaproterenol and albuterol compared with the administration of each of the beta agonists alone. Combined therapy produced significant additional improvement in FEV1 and FVC. On combined therapy, the median duration of 15% improvement in FEV1 was 5 to 7 hours, compared with 3 to 4 hours in patients receiving a beta agonist alone.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Two-year oral carcinogenicity studies in rats and mice have revealed no carcinogenic potential at dietary doses up to 6 mg/kg/day of ipratropium bromide.
- Results of various mutagenicity studies (Ames test, mouse dominant lethal test, mouse micronucleus test and chromosome aberration of bone marrow in Chinese hamsters) were negative.
- Fertility of male or female rats at oral doses up to 50 mg/kg/day was unaffected by ipratropium bromide administration. At doses above 90 mg/kg increased resorption and decreased conception rates were observed.
Clinical Studies
There is limited information regarding Ipratropium (inhalation) Clinical Studies in the drug label.
How Supplied
Ipratropium Bromide Inhalation Solution Unit Dose Vial is supplied as a 0.02% clear, colorless solution containing 2.5 mL.

Each vial is made from a low density polyethylene (LDPE) resin.
Vials are supplied in a foil pouch.
ATTENTION PHARMACIST: Detach "Patient's Instructions for Use" from Package Insert and dispense with solution.
Storage
- Store between 59°F (15°C) and 86°F (30°C).
- Protect from light.
- Store unused vials in the foil pouch.
Images
Drug Images
{{#ask: Page Name::Ipratropium (inhalation) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
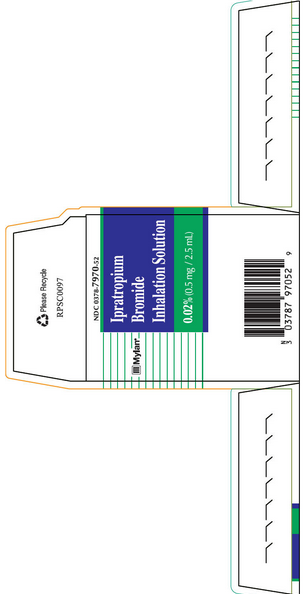
PRINCIPAL DISPLAY PANEL - 2.5 ML POUCH CARTON
Mylan®
NDC 0378-7970-52
Ipratropium Bromide Inhalation Solution
0.02% (0.5 mg / 2.5 mL)
25 x 2.5 mL Sterile Unit-Dose Vials
Rx only
FOR ORAL INHALATION ONLY


{{#ask: Label Page::Ipratropium (inhalation) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients should be advised that temporary blurring of vision, precipitation or worsening of narrow-angle glaucoma or eye pain may result if the solution comes into direct contact with the eyes. Use of a nebulizer with mouthpiece rather than face mask may be preferable, to reduce the likelihood of the nebulizer solution reaching the eyes. Patients should be advised that ipratropium bromide inhalation solution can be mixed in the nebulizer with albuterol or metaproterenol if used within one hour. Drug stability and safety of Ipratropium Bromide Inhalation Solution when mixed with other drugs in a nebulizer have not been established. Patients should be reminded that ipratropium bromide inhalation solution should be used consistently as prescribed throughout the course of therapy.



Precautions with Alcohol
Alcohol-Ipratropium (inhalation) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Ipratropium (inhalation) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Ipratropium (inhalation) Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.