Heart murmur physical examination
|
Heart murmur Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Heart murmur physical examination On the Web |
|
American Roentgen Ray Society Images of Heart murmur physical examination |
|
Risk calculators and risk factors for Heart murmur physical examination |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
The physical examination is the initial step to identify the cause and the severity of the valvular pathology causing a heart murmur. Provocative maneuvers such as hand grip (which increases systemic vascular resistance), the valsalva maneuver (which reduces return of blood to the right side of the heart), and inspiration (which increase blood return to the right side of the heart) may help to identify the underlying valvular pathology.
Systolic Murmurs
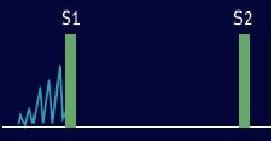
Systolic murmurs start at or after S1 and end before or at S2.
Systolic murmurs can be classified into two basic categories; ejection (midsystolic) murmurs and regurgitant murmurs. This simple classification is attractive because it has a physiologic as well as a descriptive basis [1] Systolic ejection murmurs are caused by forward flow across the LV or RV outflow tract, whereas systolic regurgitant murmurs are caused by retrograde flow from a high-pressure cardiac chamber to a low-pressure chamber.
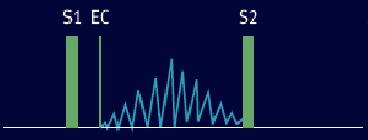
Systolic Ejection Murmurs
The systolic ejection murmur (SEM) begins shortly after the pressure in the left or right ventricle exceeds the aortic or pulmonic diastolic pressure sufficiently to open the aortic or pulmonic valve.
There is a delay between the S1, which occurs shortly after AV pressure crossover, and the beginning of the murmur. The murmur then waxes and wanes in a crescendo-decrescendo fashion often described as "diamond-shaped" or "spindle-shaped" in configuration. The murmur ends before the semilunar valve closure on the side from which it originates.
The contour of the time-intensity pattern or envelope of the murmur corresponds to the contour of the flow velocity, and the murmur is heard when the sound produced during the peak turbulence exceeds the audible threshold. Thus, not only is the overall intensity of the murmur proportional to the rate of ventricular ejection, but also its shape depends on the instantaneous flow velocity during the period of ejection.
During normal left ventricular ejection, a disproportionately large volume flow occurs in early systole. If velocity of flow exceeds the murmur threshold, a short midsystolic or ejection murmur results, and its envelope corresponds to the flow velocity pattern. If the stroke volume of the ventricle is increased, this pattern of ejection persists in an exaggerated fashion; the resulting murmur has a tendency to peak early in systole and fade out approximately halfway through the ejection phase. Such murmurs have been referred to as "kite-shaped" and are common in high-output states or conditions such as aortic regurgitation or heart block, where stroke volume is high.
The flow characteristics of normal right ventricular ejection are somewhat different. Early ejection rates are not nearly as high, and the flow curve peaks somewhat later, having a more rounded contour. This flow pattern can well explain some of the long systolic ejection murmurs heard in ASDs and the straight back syndrome, where only minimal gradients are found across the right ventricular outflow tract.
In true valvular obstruction, rapid early ejection is no longer possible; the aortic flow velocity patterns become rounded, resulting in the more symmetric murmur of Aortic Stenosis. In such cases, the instantaneous flow pattern is determined by the instantaneous pressure head with the resulting high correlation between the contour of the pressure gradient and the murmur envelope. [2]
If left ventricular or right ventricular obstruction is severe, systole is prolonged, and closure sound of the semilunar valve is delayed. The murmur, however, always stops before the closure sound on the side from which it originates, although it can envelop the closure sound of the opposite side of the circulation.
The intensity of ejection murmurs closely parallels changes in cardiac output. Any condition that increases forward flow (such as exercise, anxiety, fever, or increased stroke volume associated with the long diastolic filling period after a premature beat) increases the intensity of the murmur.
Likewise, conditions that decrease cardiac output, congestive heart failure, beta blockade, or other negative inotropic agents will decrease the intensity of the ejection murmur. Furthermore, definitive diagnosis of the systolic murmur often can be made during auscultation by careful attention to the response of the murmur to various bedside maneuvers that alter the flow and loading conditions of the heart [3] These maneuvers include respiration, the strain and release phases of the Valsalva maneuver, standing, squatting, passive leg elevation, isometric hand-grip exercise, and transient arterial occlusion.
Differential Diagnosis of Systolic Ejection Murmurs
- Benign
- Innocent systolic murmur (vibratory)
- Flow murmurs
- Hemodynamic effect (i.e., fever, hyperthyroidism, severe anemia)
- Athlete's heart
- Pathologic
Mid-systolic ejection murmurs
- A systolic ejection murmur (midsystolic) begins after the S1 and ends before A2 (in left sided pathologies) or P2 (in right sided pathologies)
Mid-systolic ejection murmurs are due to blood flow through the semilunar valves. They occur at the start of blood ejection - which starts after S1 - and ends with the cessation of the blood flow - which is before S2.
Therefore, the onset of a midsystolic ejection murmur is separated from S1 by the isovolumic contraction phase; the cessation of the murmur and the S2 interval is the aortic or pulmonary hangout time. The resultant configuration of this murmur is a crescendo-decrescendo murmur. Causes of midsystolic ejection murmurs include outflow obstruction, increased flow through normal semilunar valves, dilation of aortic root or pulmonary trunk, or structural changes in the semilunar valves without obstruction.
Obstruction to left ventricular outflow can be congenital or acquired and can be located at the valvular, supravalvular, or subvalvular level. Stenosis is occasionally present at more than one level. In the clinical evaluation, one should attempt to define the severity and the level of obstruction.
- Aortic outflow obstruction. Can be due to aortic valve stenosis or hypertrophic cardiomyopathy (HCM), with a harsh and rough quality.
- Valvular aortic stenosis can produce a harsh, or even a musical murmur over the right second intercostal space which radiates into the neck over the two carotid arteries. The most common cause of AS (Aortic Stenosis) is calcified valves due to aging followed by congenital bicuspid aortic valves (a normal aortic valve has three cusps). The distinguishing feature between these two causes is that bicuspid aortic stenosis has little or no radiation. It can be confirmed if it also has an aortic ejection sound, a short early diastolic murmur, and normal carotid pulse. The murmur in valvular AS decreases with standing and straining with Valsalva maneuver.
- Supravalvular aortic stenosis is loudest at a point slightly higher than in that of valvular AS and may radiate more to the right carotid artery.
- Subvalvular aortic stenosis is usually due to hypertrophic cardiomyopathy (HCM), with murmur loudest over the left sternal border or the apex. The murmur in HCM increases in intensity with a standing position as well as straining with Valsalva maneuver.
- Pulmonic outflow obstruction: A harsh murmur usually on left second intercostal space radiating to left neck and accompanied by palpable thrill. It can be distinguished from a VSD (Ventricular Septal Defect) by listening to the S2, which is normal in VSD but it is widely split in pulmonary stenosis. However, VSD is almost always pansystolic where the murmur of pulmonary stenosis is diamond-shaped and ends clearly before S2. Many innocent murmurs also arise from this location but S1 and S2 must split normally.
- Dilation of aortic root or pulmonary artery: Produces an ejection sound, with a short ejection systolic murmur and a relatively wide split S2. There is no hemodynamic abnormality. This is similar to pulmonary hypertension except the latter has hemodynamic instabilities.
- Increased semilunar blood flow: This can occur in situations such as anemia, pregnancy, or thyrotoxicosis.
- Aortic valve sclerosis: This is due to degenerative thickening of the roots of the aortic cusps but produces no obstruction and no hemodynamic instability and thus should be differentiated from aortic stenosis. It is heard over right second intercostal space with a normal carotid pulse and normal S2.
- Innocent midsystolic murmurs: These murmurs are not accompanied by other abnormal findings. One example is Still's murmur in children.
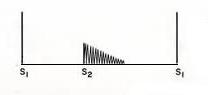
Late Systolic Murmurs
- Late systolic murmurs start after S1 and, if left sided, extends up to A2, if right sided, extends up to P2.
Late systolic murmurs are usually in a crescendo manner. Causes include mitral valve prolapse, tricuspid valve prolapse, and papillary muscle dysfunction.
- Mitral valve prolapse. This is the most common cause of late systolic murmurs. It can be heard best over the apex of the heart, usually preceded by clicks. The most common cause of mitral valve prolapse is "floppy" valve (Barlow's syndrome) syndrome. If the prolapse becomes severe enough, mitral regurgitation may occur. Any maneuver that decreases Left ventricular volume - such as standing, sitting, Valsalva maneuver, and amyl nitrate inhalation - can produce earlier onset of clicks, longer murmur duration, and decreased murmur intensity. Any maneuver that increases left ventricular volume - such as squatting, elevation of legs, hand grip, and phenylephrine - can delay the onset of clicks, shorten murmur duration, and increase murmur intensity.
- Tricuspid valve prolapse. Uncommon without concomitant mitral valve prolapse. Best heard over left lower sternal border.
- Papillary muscle dysfunction. Usually due to acute myocardial infarction or ischemia, which causes mild mitral regurgitation.
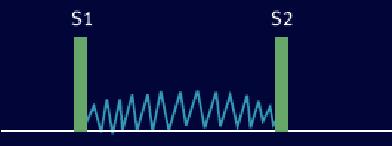
Holosystolic (Pansystolic) Murmurs
Usually due to regurgitation in cases such as mitral regurgitation, tricuspid regurgitation, or ventricular septal defect (VSD). These murmurs start at S1 and extends up to S2.
- Mitral regurgitation. In the presence of an incompetent mitral valve, the pressure in the left ventricle becomes greater than that in the left atrium at the onset of isovolumic contraction, which corresponds to the closing of the mitral valve (S1). This explains why the murmur in mitral regurgitation starts at the same time as S1. This difference in pressure extends throughout systole and can even continue after the aortic valve has closed, explaining how it can sometimes drown the sound of S2. The murmur in mitral regurgitation is high pitched and best heard at the apex with diaphragm of the stethoscope with patient in the lateral decubitus position. Left ventricular function can be assessed by determining the apical impulse. A normal or hyperdynamic apical impulse suggests good ejection fraction and primary mitral regurgitation. A displaced and sustained apical impulse suggests decreased ejection fraction and chronic and severe mitral regurgitation.
- Tricuspid insufficiency. Can be best heard over the fourth intercostal area at left sternal border. The intensity can be accentuated following inspiration (Carvallo's sign) due to increased regurgitant flow in right ventricular volume. Tricuspid regurgitation is most often secondary to pulmonary hypertension. Primary tricuspid regurgitation is less common and can be due to bacterial endocarditis following IV drug use, Ebstein's anomaly, carcinoid disease, or prior right ventricular infarction.
- Ventricular septal defect. VSD is a defect in the ventricular wall, producing a shunt between the left and right ventricles. Since the left ventricle has a higher pressure than the right ventricle, flow during systole occurs from the left to right ventricle, producing the holosystolic murmur. It can be best heard over the left third and fourth intercostal spaces and along the sternal border. It is associated with normal pulmonary artery pressure and thus S2 is normal. This fact can be used to distinguish from pulmonary stenosis, which has a wide splitting S2. When the shunt becomes reversed ("Eisenmenger's syndrome"), the murmur may be absent and S2 can become markedly accentuated and single.
Example of Holosystolic Murmur: VSD Murmur
Innocent Murmurs
Innocent murmurs are always systolic ejection in nature and occur without evidence of physiologic or structural abnormalities in the cardiovascular system when peak flow velocity in early systole exceeds the murmur threshold.
These murmurs are almost always less than grade 3 in intensity and vary considerably from examination to examination and with body position and level of physical activity. They are not associated with a thrill or with radiation to the carotid arteries or axilla. They can arise from flow across either the normal left ventricular or right ventricular outflow tract and always end well before semilunar valve closure.
Innocent murmurs are found in approximately 30-50 % of all children. In young children, especially children 3 to 8 years of age, the vibratory systolic (Still's murmur) murmur is common. It has a very distinctive quality described as "groaning," "croaking," "buzzing," or "twanging." It is heard best along the left sternal border at the third or fourth intercostal space and disappears by puberty. Regardless of the exact cause, most authorities agree that this murmur originates from flow in the left ventricular outflow tract.
Innocent systolic ejection murmurs also have been attributed to flow in the normal right ventricular outflow tract and have been termed innocent pulmonic systolic murmurs because the site of their maximal intensity is auscultated best in the pulmonic area at the second left intercostal space with radiation along the left sternal border. These are low to medium in pitch, with a blowing quality, and are common in children, adolescents, and young adults. Stein and coworkers [4] who used high-fidelity catheter-tipped micromanometers to record intracardiac sound and pressure in the aorta and pulmonary artery in adults with normal valves, invariably recorded the ejection murmur in the region of the aortic valve. They concluded that these murmurs, despite their pulmonic precordial location, were aortic in origin.
In adults older than 50 years of age, innocent murmurs caused by flow in the left ventricular outflow tract are often heard and can be of a higher frequency, with a musical quality, and frequently loudest at the apex. They can be associated with a tortuous, dilated sclerotic aortic root, often in the setting of systolic hypertension. Mild sclerosis of the aortic valve also can be present.
The preceding descriptive breakdown of innocent murmurs is based primarily on age, precordial location, and distinctive acoustic qualities. Because both innocent and pathologic ejection murmurs have the same mechanism of production, it is "the company the murmur keeps" that affords the differential diagnosis of the pathologic systolic ejection murmur from the innocent murmur.[5]
For a murmur to be considered innocent, the examination of the cardiovascular system must disclose no abnormalities. Blood pressure and contour of the carotid, femoral, and brachial arteries always should be evaluated carefully. There should be no elevation of the jugular venous pressure, and the contour of the jugular pulse should be normal, without exaggeration of either the a or v wave. Evidence of cardiac enlargement on physical examination should be absent, and palpation of the apex in the left lateral position should show no evidence of a presystolic impulse, sustained systolic motion, or hyperdynamic circulation.
On auscultation, normal physiologic splitting should be present. A physiologic S3 is often present in association with an innocent murmur in children and young adults but should not be heard after age 30.
An S4 is rarely heard in normal children and adults (younger than 50 years of age) and always should be considered to be abnormal when associated with a palpable presystolic impulse. Systolic ejection sounds of valvular origin as well as mid-systolic non ejection sounds should be absent because their presence points to minor abnormalities of the semilunar and AV valves, respectively. The remainder of the physical examination should show no evidence of a cardiac cause of pulmonary or systemic congestion.
The supraclavicular arterial murmur or bruit is a common finding in normal individuals, particularly children and adolescents. These murmurs are maximal in intensity above the clavicles and tend to be louder on the right, although they are often heard bilaterally. The bruit begins shortly after S1, is diamond-shaped, and is of brief duration, usually occupying less than half of systole. Although the exact mechanism is unknown, it is related to peak flow velocity near the origin of the normal subclavian, brachiocephalic or carotid artery.
Unlike the cardiac ejection murmur, the supraclavicular murmur is always louder above the clavicles than below them. Complete compression of the subclavian artery can cause the murmur to disappear completely, whereas partial compression occasionally can intensify it. Hyperextension of the shoulders is a simple bedside maneuver that can decrease the intensity of the murmur and cause it to disappear completely. In the adult, the supraclavicular murmur must be distinguished from the murmur of true organic carotid obstruction, this latter murmur being longer, often extending through S2, and frequently associated with a history suggestive of transient ischemic attacks.
Functional Systolic Ejection Murmurs
Systolic ejection murmurs produced by high cardiac output states are functional and flow-related but are excluded from the category of innocent murmurs because of their associated altered physiologic state. These include the cardiac murmurs of thyrotoxicosis, pregnancy, anemia, fever, exercise, and peripheral arteriovenous fistula, which are best interpreted in light of the total presentation of the patient. Although these murmurs are often grade 3 and occasionally grade 4 in intensity, they always end well before S2 and are only rarely confused with obstruction of the left ventricular or right ventricular outflow tract. The large stroke volume associated with high-degree heart block often produces a functional systolic murmur; when found in the setting of complete heart block, beat-to-beat variations in the intensity of the murmur are present as a result of the random contribution of atrial systole to left ventricular filling.
The functional systolic murmur in patients with a hemodynamically significant ASD is caused by the increased flow in the right ventricular outflow tract secondary to the left-to-right shunt at the atrial level. It is easily diagnosed at the bedside "by the company it keeps." The hallmark of this condition is wide, fixed splitting of S2. When the shunt is large (more than 2.5:1), a hyperdynamic parasternal impulse is usually present, and a diastolic flow rumble is often heard in the tricuspid area. In addition, the tricuspid closure is loud, and prominent a and v waves are seen in the jugular venous pressure.
An important condition to be differentiated from an ASD is narrowing of the anteroposterior diameter of the bony thorax. Prominent systolic murmurs (often grade 3 or 4 are heard in patients who have the straight back syndrome and/or pectus excavatum).
Audible expiratory splitting is frequently present and, coupled with a prominent pulmonary artery on the chest x-ray (secondary to the narrow anteroposterior [AP] diameter), can lead to additional unnecessary procedures to rule out an ASD. Careful attention at the bedside to the physical examination of the spine, thoracic cage, and sternum should be part of the routine evaluation of any patient with a murmur. Often, confirmation of the thoracic abnormality with a lateral chest film is all that is necessary for definitive evaluation.
Prominent systolic ejection murmurs are the rule in patients with significant aortic regurgitation secondary to the large forward stroke volume. Although no significant left ventricular outflow pressure gradient is found in these patients, the intensity of such murmurs can be grade 4 or 5, and occasionally they are associated with a thrill. They always end well before aortic closure and are clearly separated from the early regurgitant murmur. Such a murmur is rarely confused with significant valvular obstruction because of the peripheral findings of wide-open aortic regurgitation. When true valvular obstruction is present (mixed aortic stenosis and aortic regurgitation), the longer systolic ejection murmur is often associated with a prominent thrill. Systolic ejection murmurs caused by large right ventricular stroke volume are also seen in severe organic pulmonic valvular regurgitation.
Ventricular ejection into a dilated great vessel is commonly associated with a systolic ejection murmur. In the elderly, such murmurs are caused by ejection into a dilated, sclerotic aorta and often are best heard at the apex. Frequently, degenerative changes of the aortic valve are also present, and the clinician is faced with a difficult decision as to whether or not true obstruction exists. The presence of significant calcification on fluoroscopic examination favors true obstruction and can be confirmed when a significant gradient is demonstrated by Doppler studies. A systolic ejection murmur caused by right ventricle ejection into a massively dilated pulmonary artery is frequently present in idiopathic dilatation of the pulmonary artery, which is often confused with an ASD as a result of the wide auditory expiratory splitting present in this condition.
Short systolic ejection murmurs, frequently associated with a prominent late pulmonary ejection sound, are also seen in dilated pulmonic arteries secondary to severe pulmonary hypertension of any cause. Physical findings of severe pulmonary hypertension are always present, including a prominent parasternal impulse and increased intensity of the pulmonic component of S2, which is well heard at the apex. Prominent a waves in the neck and a right-sided S4 that increases with inspiration are present if the ventricular septum is intact. If the PH is associated with intracardiac shunting, cyanosis frequently is present. A high-pitched, early diastolic murmur of pulmonic regurgitation secondary to severe pulmonary hypertension often is present.
Diastolic murmurs
Diastolic murmurs start at or after S2 and ends before or at S1.
Early Diastolic Murmurs
They start at the same time as S2 with the close of the semilunar valves and typically ends before S1.
Common causes include aortic or pulmonary regurgitation and Left anterior descending artery stenosis.
Aortic regurgitation. The murmur is low intensity, high-pitched, best heard over the left sternal border or over the right second intercostal space, especially if the patient leans forward and holds breath in full expiration. The radiation is typically toward the apex. The configuration is usually decrescendo and has a blowing character. The presence of this murmur is a good positive predictor for aortic regurgitation and the absence of this murmur strongly suggests the absence of aortic regurgitation. An Austin Flint murmur is usually associated with significant aortic regurgitation.
Pulmonary regurgitation. Pulmonary regurgitation murmur is most commonly due to pulmonary hypertension (Graham-Steell murmur). It is a high-pitched and blowing murmur with a decrescendo configuration. It may increase in intensity during inspiration and best heard over left second and third intercostal spaces. The murmur usually does not extend to S1.
Left anterior descending artery stenosis. This murmur, also known as Dock's murmur, is similar to that of aortic regurgitation and is heard at the left second or third intercostal space. A Coronary artery bypass surgery can eliminate the murmur.
-
Early diastolic murmur: Aortic Insufficiency
-
Smooth A2 and early diastolic murmur: Aortic Insufficiency
Mid Diastolic Murmurs
Mitral stenosis. This murmur has a rumbling character and is best heard with the bell of the stethoscope in the left ventricular impulse area with the patient in the lateral decubitus position. It usually starts with an opening snap. In general, the longer the duration, the more severe the mitral stenosis. However, this rule can be misleading in situations where the stenosis is so severe that the flow becomes reduced, or during high-output situations such as pregnancy where a less severe stenosis may still produce a strong murmur.
Tricuspid stenosis. Best heard over the left sternal border with rumbling character and tricuspid opening snap with wide splitting S1. May increase in intensity with inspiration (Carvallo's sign). Tricuspid stenosis often occurs in association with mitral stenosis. Isolated tricuspid stenosis are often associated with carcinoid disease and right atrial myxoma.
Atrial myxoma. Atrial myxomas are benign tumors of the heart. Left myxomas are far more common than right myxomas and those may cause obstruction of the mitral valve producing a mid-diastolic murmur similar to that of mitral stenosis. An echocardiographic evaluation is necessary.
Increased flow across the atrioventricular valve. This can also produce a mid-diastolic murmur, such as in severe mitral regurgitation where a large regurgitant volume in the left atrium can lead to functional mitral stenosis.
Austin Flint murmur. An apical diastolic rumbling murmur in patients with pure aortic regurgitation. This can be mistaken with the murmur in mitral stenosis and should be noted by the fact that an Austin Flint murmur does not have an opening snap that is found in mitral stenosis.
Carey-Coombs murmur. A mid-diastolic murmur over the left ventricular impulse due to mitral valvulitis from acute rheumatic fever.
-
Mid-diastolic (starts with mitral opening snap) and pre-systolic murmur (Late diastolic): Mitral Stenosis
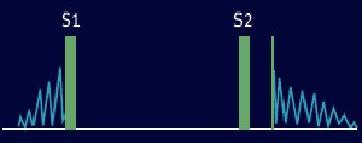
Late Diastolic (Presystolic) Murmurs
A late diastolic (presystolic) murmur starts well after the second heart sound (S2) and extends up to the mitral component (left sided) or to the tricuspid component (right sided) of S1.
These murmurs start after S2 and extends up to S1 and have a crescendo configuration. They include mitral stenosis, tricuspid stenosis, myxoma, and complete heart block.
- Mitral stenosis
- In complete heart block. A short late diastolic murmur can occasionally be heard (Rytand's murmur). The precise mechanism of Rytand's murmur has not been elucidated; diastolic mitral regurgitation has been postulated [6]
- Left-to-right shunts
- Tricuspid stenosis
- Myxoma
Continuous murmurs
These murmurs are due to blood flow from a high pressure chamber or vessel to a lower pressure system [8]
- Patent ductus arteriosus. PDA is an abnormal connection between the aorta and the pulmonary artery, which normally should be closed in infancy. Since aortic pressure is higher than pulmonary pressure, a continuous murmur occurs, which is often described as a machinery murmur, or Gibson's murmur.
- Venous hum: frequently heard in children over the base of the neck, usually best on the right side. Louder in diastole and disappears in the supine position or with compression.
- Mammary Souffle: heard during late pregnancy and the early postpartum period in lactating women. It is thought to be arterial in origin and can be bilateral. Is louder, peaks in systole, vanishes in the upright position, and is abolished by local compression.
- Aorticopulmonary Window: is a rare congenital opening between the aorta and the pulmonary trunk just above the aortic valve. It is associated with other abnormalities in approximately 1/2 the cases, such as anomalous origin of the coronary arteries from the pulmonary trunk and coarctation of the aorta. The murmur is lower and more medial in location. In adults is presented without a murmur and clinical features of the Eisenmenger's syndrome.
- Rupture of the Sinus of Valsalva: It can rupture into a cardiac chamber. Almost always arise from the right or the non coronary cusps and rupture into the RV and RA respectively. Occasionally is acquired as a result of endocarditis. Large acute perforations tend to occur between puberty and age 30 causing severe retrosternal chest pain, dyspnea related to the large left to right shunt.The murmur is louder in a lower parasternal position. People with VSDs and sudden development of chest pain have frequently experienced rupture of a coexistent sinus of valsalva aneurysm. A rupture of the sinus of valsalva can distort or compress the coronary arteries and cause an infarction, distort the conduction system, cause AV block, distort the aortic valve, and cause AS or AI. Patients with rupture of the sinus of valsalva, should undergo surgical correction because mortality is high within a year of rupture.
- Fistulas of the coronary circulation: Generally a coronary artery that arises normally will communicate with the RV. Occasionally drain into the pulmonary trunk. The artery that forms the fistula is generally dilated, elongated, and tortuous. The left to right shunt is small. It may not be recognized radiographically. Patients with small fistula are generally asymptomatic. Therefore, no justification to repair it. On the other hand, if the shunt is extremely large, then failure may develop in the 4th, 5th or 6th decade of life. It can be treated with ligation.
- Anomalous Origin of the Coronary Artery From the Pulmonary Trunk: Usually refers to the origin of the left coronary artery from the pulmonary trunk. Approximately, 80 to 90% of the patients die in their first year of life due to ischemia. Blood from the high pressure RCA flows to the low pressure left coronary artery and the pulmonary artery. Anomalous origin of the RCA from the PA is much rarer, but these patients stand a better chance of surviving into adulthood because it is less likely to cause ischemia early in life.
- Pulmonary Arteriovenous Fistulas: Instead of being localized to the precordium, these murmurs are localized to the lung fields. Cyanosis is presented with a normal heart size. Seen in Rendu-Osler-Weber syndrome. A fistula causing cyanosis could be treated with lobectomy if it is confined to a single lobe.
- Shunts. Usually a left to right shunt through a small atrial septal defect in the presence of mitral valve obstruction.[9]
Interventions that change murmur characteristics
- Inspiration will increase the amount of blood filling into the right ventricle, thereby prolonging ejection time. This will affect the closure of the pulmonary valve. This finding, also called Carvallo's maneuver has been found by studies to have a sensitivity of 100% and a specificity of 80% to 88% in detecting murmurs originating in the right heart [10] [11].
- Abrupt standing reduces ventricular filling and increases murmurs such as hypertrophic obstructive cardiomyopathy (HOCM) and reduces murmurs such as aortic stenosis
- Squatting
- Valsalva maneuver reduces blood return to the right heart, and reduces ventricular filling. One study found the valsalva maneuver to have a sensitivity of 65%, specificity of 96% in detecting Hypertrophic obstructive cardiomyopathy (HOCM) [10].
- Isometric hand grip increases systemic vascular resistance
- Post ectopic potentiation increases left ventricular contraction which increases the murmur of aortic stenosis, and narrows the pulse pressure in hypertrophic obstructive cardiomyopathy (HOCM)
- Amyl nitrite
- Methoxamine
- Positioning the patient in the left lateral position increases the intensity of a mitral murmur in the mitral area
Other Physical Examination Findings in Individuals with Heart Murmurs
- Jugular venous distension may be observed in tricuspid valve disease (tricuspid regurgitation and tricuspid stenosis)
- Bruits may reflect transmitted murmurs from aortic stenosis. In acquired aortic stenosis, the transmission is bilateral, and in bicuspid aortic stenosis it may be unilateral.
- The carotid upstroke will be diminished in aortic stenosis (pulsus parvus and tardus)
- Peripheral pulses may be accentuated in the presence of aortic insufficiency
- Peripheral edema can be seen if there is right heart failure or constrictive pericarditis
References
- ↑ Mangione S, Nieman LZ. Cardiac auscultatory skills of internal medicine and family practice trainees. A comparison of diagnostic proficiency. JAMA. 1997; 278(9):717. PMID 9286830
- ↑ Murgo JP. Systolic ejection murmurs in the era of modern cardiology. What do we really know? J Am Coll Cardiol. 1998; 32: 1596. PMID 9822084
- ↑ Gallavardin L, Ravault P. Le souffle du retrecissement aortique puet changer de timbre et devenir musical dans sa propagation apexienne. Lyon Med. 1925;135:523–529.
- ↑ Stein PD, Sabbah HN. Aortic origin of innocent murmurs. Am J Cardiol. 1977;39:665–671. PMID 67794
- ↑ Murgo JP, Altobelli SA, Dorethy JF, et al. Normal ventricular ejection dynamics in man during rest and exercise. In: Leon DF, Shaver JA, eds. Physiologic Principles of Heart Sounds and Murmurs (Monograph 46). New York: American Heart Association; 1975:92–101
- ↑ Rutishauser, W, Wirz, P, Gander, M, et al. Atriogenic diastolic influx in patients with atrioventricular heart block. Circulation 1966; 34:807.
- ↑ Panidis, IP, Ross, J, Munley, B, et al. Diastolic mitral regurgitation in patients with atrioventricular conduction abnormalities: A common finding by Doppler echocardiography. J Am Coll Cardiol 1986; 7:768.
- ↑ Craige, E, Milward, DK. Diastolic and continuous murmurs. Prog Cardiovasc Dis 1971; 14:38.
- ↑ Giuliani et al, Cardiology: Fundamentals and Practice, Second Edition, Mosby Year Book, Boston, 1991, pp. 1653-1663.
- ↑ 10.0 10.1 Lembo N, Dell'Italia L, Crawford M, O'Rourke R (1988). "Bedside diagnosis of systolic murmurs". N Engl J Med. 318 (24): 1572–8. PMID 2897627.
- ↑ Maisel A, Atwood J, Goldberger A (1984). "Hepatojugular reflux: useful in the bedside diagnosis of tricuspid regurgitation". Ann Intern Med. 101 (6): 781–2. PMID 6497192.