Ergotamine and caffeine (rectal)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Adeel Jamil, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
* Serious and/or life-threatening peripheral ischemia has been associated with the coadministration of ergotamine tartrate and caffeine with potent CYP 3A4 inhibitors including protease inhibitors and macrolide antibiotics. Because CYP 3A4 inhibition elevates the serum levels of ergotamine tartrate and caffeine, the risk for vasospasm leading to cerebral ischemia and/or ischemia of the extremities is increased. Hence, concomitant use of these medications is contraindicated.
|
Overview
Ergotamine and caffeine (rectal) is an ergot alkaloid, antimigraine and central nervous system agent that is FDA approved for the treatment of to abort or prevent vascular headache, e.g., migraine, migraine variants or so-called “histaminic cephalalgia”. There is a Black Box Warning for this drug as shown here. Common adverse reactions include pruritus, nausea and vomiting, muscle weakness, numbness, paresthesia, visual disturbance, vertigo, localized edema and itching.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Indicated as therapy to abort or prevent vascular headache, e.g., migraine, migraine variants or so-called “histaminic cephalalgia”.
Dosing Information
Procedure
- For best results, dosage should start at the first sign of an attack.
p
- Early Administration Gives Maximum Effectiveness
Maximum Adult Dosage
- Rectally
- Two suppositories is the maximum dose for an individual attack.
- Total weekly dosage should not exceed 5 suppositories. Ergotamine Tartrate and Caffeine Suppositories should not be used for chronic daily administration.
- In carefully selected patients, with due consideration of maximum dosage recommendations, administration of the drug at bedtime may be an appropriate short-term preventive measure.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ergotamine and caffeine (rectal) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ergotamine and caffeine (rectal) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Ergotamine and caffeine (rectal) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ergotamine and caffeine (rectal) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ergotamine and caffeine (rectal) in pediatric patients.
Contraindications
- Coadministration of ergotamine with potent CYP 3A4 inhibitors (ritonavir, nelfinavir, indinavir, erythromycin, clarithromycin, and troleandomycin) has been associated with acute ergot toxicity (ergotism) characterized by vasospasm and ischemia of the extremities (see PRECAUTIONS: DRUG INTERACTIONS), with some cases resulting in amputation. There have been rare reports of cerebral ischemia in patients on protease inhibitor therapy when ergotamine tartrate and caffeine was coadministered, at least one resulting in death. Because of the increased risk for ergotism and other serious vasospastic adverse events, ergotamine use is contraindicated with these drug and other potent inhibitors of CYP 3A4 (e.g., ketoconazole, itraconazole) (see WARNINGS: CYP 3A4 INHIBITORS).
- Ergotamine tartrate and caffeine may cause fetal harm when administered to pregnant women. Ergotamine tartrate and caffeine is contraindicated in women who are or may become pregnant. If this drug is used during pregnancy or if the patient becomes pregnant while taking this product, the patient should be apprised of the potential hazard to the fetus.
- Peripheral vascular disease, coronary heart disease, hypertension, impaired hepatic or renal function and sepsis.
- Hypersensitivity to any of the components.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
* Serious and/or life-threatening peripheral ischemia has been associated with the coadministration of ergotamine tartrate and caffeine with potent CYP 3A4 inhibitors including protease inhibitors and macrolide antibiotics. Because CYP 3A4 inhibition elevates the serum levels of ergotamine tartrate and caffeine, the risk for vasospasm leading to cerebral ischemia and/or ischemia of the extremities is increased. Hence, concomitant use of these medications is contraindicated.
|
CYP 3A4 Inhibitors (e.g. Macrolide Antibiotics and Protease Inhibitors)
- Coadministration of ergotamine with potent CYP 3A4 inhibitors such as protease inhibitors or macrolide antibiotics has been associated with serious adverse events, for this reason, these drugs should not be given concomitantly with ergotamine (see CONTRAINDICATIONS). While these reactions have not been reported with less potent CYP 3A4 inhibitors, there is a potential risk for serious toxicity including vasospasm when these drugs are used with ergotamine. Examples of less potent CYP 3A4 inhibitors include: saquinavir, nefazodone, fluconazole, fluoxetine, grapefruit juice, fluvoxamine, zileuton, metronidazole, and clotrimazole. These lists are not exhaustive, and the prescriber should consider the effects on CYP3A4 of other agents being considered for concomitant use with ergotamine.
Fibrotic Complications
- There have been a few reports of patients on ergotamine tartrate and caffeine therapy developing retroperitoneal and/or pleuropulmonary fibrosis. There have also been rare reports of fibrotic thickening of the aortic, mitral, tricuspid, and/or pulmonary valves with long-term continuous use of ergotamine tartrate and caffeine. Ergotamine tartrate should not be used for chronic daily administration.
PRECAUTIONS
General
- Although signs and symptoms of ergotism rarely develop even after long term intermittent use of the rectally administered drug, care should be exercised to remain within the limits of recommended dosage.
- Ergotism is manifested by intense arterial vasoconstriction, producing signs and symptoms of peripheral vascular ischemia. Ergotamine induces vasoconstriction by a direct action on vascular smooth muscle. In chronic intoxication with ergot derivatives, headache, intermittent claudication, muscle pains, numbness, coldness and pallor of the digits may occur. If the condition is allowed to progress untreated, gangrene can result.
- While most cases of ergotism associated with ergotamine treatment result from frank overdosage, some cases have involved apparent hypersensitivity. There are few reports of ergotism among patients taking doses within the recommended limits or for brief periods of time. In rare instances, patients, particularly those who have used the medication indiscriminately over long periods of time, may display withdrawal symptoms consisting of rebound headache upon discontinuation of the drug.
- Rare cases of solitary rectal or anal ulcer have occurred from abuse of ergotamine suppositories usually in higher than recommended doses or with continual use at the recommended dose for many years. Spontaneous healing occurs within usually 4-8 weeks after drug withdrawal.
Drug abuse and dependence
- There have been reports of drug abuse and psychological dependence in patients on ergotamine tartrate and caffeine therapy. Due to chronicity of vascular headaches, it is imperative that patients be advised not to exceed recommended dosages with long-term use to avoid ergotism.
Adverse Reactions
Clinical Trials Experience
Cardiovascular:
- Vasoconstrictive complications of a serious nature may occur at times. These include ischemia, cyanosis, absence of pulse, cold extremities, gangrene, precordial distress and pain, EKG changes and muscle pains. Although these effects occur most commonly with long-term therapy at relatively high doses, they have also been reported with short-term or normal doses. Other cardiovascular adverse effects include transient tachycardia or bradycardia and hypertension.
Gastrointestinal:
- Nausea and vomiting; rectal or anal ulcer (from overuse of suppositories).
Neurological:
- Paresthesias, numbness, weakness, and vertigo.
Allergic:
- Localized edema and itching.
Fibrotic Complications:
- Retroperitoneal pleuropulmonary fibrosis, fibrotic thickening of the aortic, mitral, tricuspid, and/or pulmonary valves with long-term continuous use of ergotamine tartrate and caffeine.
Postmarketing Experience
There is limited information regarding Ergotamine and caffeine (rectal) Postmarketing Experience in the drug label.
Drug Interactions
CYP 3A4 Inhibitors (e.g. Macrolide Antibiotics and Protease Inhibitors)
- Ergotamine tartrate and caffeine should not be administered with other vasoconstrictors. Use with sympathominetics (pressor agents) may cause extreme elevation of blood pressure. The beta-blocker Inderal (propranolol) has been reported to potentiate the vasoconstrictive action of ergotamine tartrate and caffeine by blocking the vasodilating property of epinephrine. Nicotine may provoke vasoconstriction in some patients, predisposing to a greater ischemic response to ergot therapy.
- The blood levels of ergotamine-containing drugs are reported to be elevated by the concomitant administration of macrolide antibiotics and vasospastic reactions have been reported with therapeutic doses of the ergotamine-containing drugs when coadministered with those antibiotics.
Use in Specific Populations
Pregnancy
Teratogenic Effects
- There are no studies on the placental transfer or teratogenicity of the combined products of ergotamine tartrate and caffeine. Caffeine is known to cross the placenta and has been shown to be teratogenic in animals. Ergotamine crosses the placenta in small amounts, although it does not appear to be embryotoxic in this quantity. However, prolonged vasoconstriction of the uterine vessels and/or increased myometrial tone leading to reduced myometrial and placental blood flow may have contributed to fetal growth retardation observed in animals.
Nonteratogenic Effects
- Ergotamine tartrate and caffeine is contraindicated in pregnancy due to the oxytocic effects of ergotamine.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ergotamine and caffeine (rectal) in women who are pregnant.
Labor and Delivery
- Ergotamine tartrate and caffeine is contraindicated in labor and delivery due to its oxytocic effect which is maximal in the third trimester.
Nursing Mothers
- Ergot drugs are known to inhibit prolactin but there are no reports of decreased lactation with ergotamine tartrate and caffeine. Ergotamine is excreted in breast milk and may cause symptoms of vomiting, diarrhea, weak pulse and unstable blood pressure in nursing infants. Because of the potential for serious adverse reactions in nursing infants from ergotamine tartrate and caffeine, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
There is no FDA guidance on the use of Ergotamine and caffeine (rectal) in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Ergotamine and caffeine (rectal) in geriatric settings.
Gender
There is no FDA guidance on the use of Ergotamine and caffeine (rectal) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ergotamine and caffeine (rectal) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Ergotamine and caffeine (rectal) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Ergotamine and caffeine (rectal) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Ergotamine and caffeine (rectal) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Ergotamine and caffeine (rectal) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Rectal
Monitoring
There is limited information regarding Ergotamine and caffeine (rectal) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Ergotamine and caffeine (rectal) and IV administrations.
Overdosage
- The toxic effects of an acute overdosage of ergotamine tartrate and caffeine are due primarily to the ergotamine component. The amount of caffeine is such that its toxic effects will be overshadowed by those of ergotamine. Symptoms include vomiting, numbness, tingling, pain and cyanosis of the extremities associated with diminished or absent peripheral pulses; hypertension or hypotension; drowsiness, stupor, coma, convulsions and shock. A case has been reported of reversible bilateral papillitis with ring scotomata in a patient who received five times the recommended daily adult dose over a period of 14 days.
- Treatment consists of removal of the offending drug by enema. Maintenance of adequate pulmonary ventilation, correction of hypotension, and control of convulsions and blood pressure are important considerations. Treatment of peripheral vasospasm should consist of warmth, but not heat, and protection of the ischemic limbs. Vasodilators may be beneficial but caution must be exercised to avoid aggravating an already existent hypotension.
Pharmacology
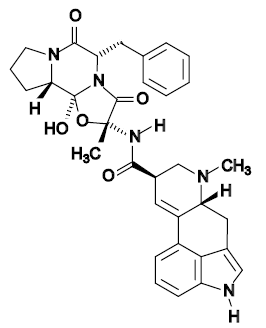
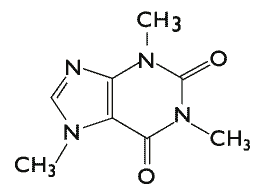
Mechanism of Action
- Ergotamine is an alpha adrenergic blocking agent with a direct stimulating effect on the smooth muscle of peripheral and cranial blood vessels and produces depression of central vasomotor centers. The compound also has the properties of serotonin antagonism. In comparison to hydrogenated ergotamine, the adrenergic blocking actions are less pronounced and vasoconstrictive actions are greater.
- Caffeine, also a cranial vasoconstrictor, is added to further enhance the vasoconstrictive effect without the necessity of increasing ergotamine dosage.
Structure
Ergotamine Tartrate and Caffeine Suppository
- Ergotamine tartrate USP 2 mg
- Caffeine USP 100 mg
- Inactive Ingredients: tartaric acid NF, and hard fat NF
- Ergotamine Tartrate and Caffeine Suppositories are for rectal administration only.
- Ergotamine Tartrate and Caffeine Suppositories are sealed in foil to afford protection from leakage. If an unavoidable period of exposure to heat softens the suppository, it should be chilled in ice-cold water to solidify it before removing the foil.
Pharmacodynamics
There is limited information regarding Ergotamine and caffeine (rectal) Pharmacodynamics in the drug label.
Pharmacokinetics
- Many migraine patients experience excessive nausea and vomiting during attacks, making it impossible for them to retain any oral medication. In such cases, therefore, the only practical means of medication is through the rectal route where medication may reach the cranial vessels directly, evading the splanchnic vasculature and the liver.
Pharmacokinetics: Interactions
- Pharmacokinetic interactions (increased blood levels of ergotamine) have been reported in patients treated orally with ergotamine and macrolide antibiotics (e.g., troleandomycin, clarithromycin, erythromycin), and in patients treated orally with ergotamine and protease inhibitors (e.g. ritonavir) presumably due to inhibition of cytochrome P450 3A metabolism of ergotamine (See CONTRAINDICATIONS). Ergotamine has also been shown to be an inhibitor of cytochrome P450 3A catalyzed reactions. No pharmacokinetic interactions involving other cytochrome P450 isoenzymes are known.
Nonclinical Toxicology
There is limited information regarding Ergotamine and caffeine (rectal) Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Ergotamine and caffeine (rectal) Clinical Studies in the drug label.
How Supplied
Ergotamine Tartrate and Caffeine Suppositories USP are supplied in boxes of 12 foil wrapped suppositories.
NDC 60809-166-12.
Manufactured for: Crealta Pharmaceuticals LLC Rev. 03/14 Glendale, WI 53217 8-0166CRA1
L166PI.00 03/14
Storage
- The suppositories should be refrigerated at 2°-8°C (36°-46°F).
Images
Drug Images
{{#ask: Page Name::Ergotamine and caffeine (rectal) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
NDC 60809-166-12
Crealta Pharmaceuticals
MIGERGOT®
Ergotamine Tartrate and Caffeine Suppositories USP
Each Rectal Suppository Contains: Ergotamine Tartrate, USP 2 mg, Caffeine, USP 100 mg, Tartaric Acid 21.5 mg
Usual Adult Dose: See Package insert. Refrigerate at 2° - 8°C (36°-46°F)
12 Rectal Suppositories
Rx Only

{{#ask: Label Page::Ergotamine and caffeine (rectal) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients should be advised that one suppository of ergotamine tartrate and caffeine should be taken at the first sign of a migraine headache. No more than 2 suppositories should be taken for any single migraine attack. No more than 5 suppositories should be taken during any 7-day period. Administration of Ergotamine Tartrate and Caffeine Suppositories should not exceed the dosing guidelines and should not be used for chronic daily administration. Ergotamine tartrate and caffeine should be used only for migraine headaches. It is not effective for other types of headaches and it lacks analgesic properties. Patients should be advised to report to the physician immediately any of the following: numbness or tingling in the fingers or toes, muscle pain in the arms and legs, weakness in the legs, pain in the chest or temporary speeding or slowing of the heart rate, swelling or itching.
Precautions with Alcohol
Alcohol-Ergotamine and caffeine (rectal) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Ergotamine and caffeine (rectal) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Ergotamine and caffeine (rectal) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.