Divalproex sodium adverse reactions
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Adverse Reactions
The following Adverse Reactions are discussed in greater detail in other sections of the labeling.
- Hepatic failure (5.1)
- Birth defects (5.2)
- Decreased IQ following in utero exposure (5.3)
- Pancreatitis (5.5)
- Thrombocytopenia (5.9)
- Hyperammonemic encephalopathy (5.10, 5.11)
- Multi-organ hypersensitivity reactions (5.13)
- Somnolence in the elderly (5.15)
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
6.1 Mania
The incidence of treatment-emergent events has been ascertained based on combined data from two three week placebo-controlled clinical trials of Depakote in the treatment of manic episodes associated with bipolar disorder. The adverse reactions were usually mild or moderate in intensity, but sometimes were serious enough to interrupt treatment. In clinical trials, the rates of premature termination due to intolerance were not statistically different between placebo, Depakote, and lithium carbonate. A total of 4%, 8% and 11% of patients discontinued therapy due to intolerance in the placebo, Depakote, and lithium carbonate groups, respectively.
Table 2 summarizes those adverse reactions reported for patients in these trials where the incidence rate in the Depakote-treated group was greater than 5% and greater than the placebo incidence, or where the incidence in the Depakote-treated group was statistically significantly greater than the placebo group. Vomiting was the only reaction that was reported by significantly (p ≤ 0.05) more patients receiving Depakote compared to placebo.
 |
The following additional adverse reactions were reported by greater than 1% but not more than 5% of the 89 Depakote-treated patients in controlled clinical trials:
- Body as a Whole: Chest pain, chills, chills and fever, fever, neck pain, neck rigidity.
- Cardiovascular System: Hypertension, hypotension, palpitations, postural hypotension, tachycardia, vasodilation.
- Digestive System: Anorexia, fecal incontinence, flatulence, gastroenteritis, glossitis, periodontal abscess.
- Hemic and Lymphatic System: Ecchymosis.
- Metabolic and Nutritional Disorders: Edema, peripheral edema.
- Musculoskeletal System: Arthralgia, arthrosis, leg cramps, twitching.
- Nervous System: Abnormal dreams, abnormal gait, agitation, ataxia, catatonic reaction, confusion, depression, diplopia, dysarthria, hallucinations, hypertonia, hypokinesia, insomnia, paresthesia, reflexes increased, tardive dyskinesia, thinking abnormalities, vertigo.
- Respiratory System: Dyspnea, rhinitis.
- Skin and Appendages: Alopecia, discoid lupus erythematosus, dry skin, furunculosis, maculopapular rash, seborrhea.
- Special Senses: Amblyopia, conjunctivitis, deafness, dry eyes, ear pain, eye pain, tinnitus.
- Urogenital System: Dysmenorrhea, dysuria, urinary incontinence.
6.2 Epilepsy
Based on a placebo-controlled trial of adjunctive therapy for treatment of complex partial seizures, Depakote was generally well tolerated with most adverse reactions rated as mild to moderate in severity. Intolerance was the primary reason for discontinuation in the Depakote-treated patients (6%), compared to 1% of placebo-treated patients. Table 3 lists treatment-emergent adverse reactions which were reported by ≥ 5% of Depakote-treated patients and for which the incidence was greater than in the placebo group, in the placebo-controlled trial of adjunctive therapy for treatment of complex partial seizures. Since patients were also treated with other antiepilepsy drugs, it is not possible, in most cases, to determine whether the following adverse reactions can be ascribed to Depakote alone, or the combination of Depakote and other antiepilepsy drugs.
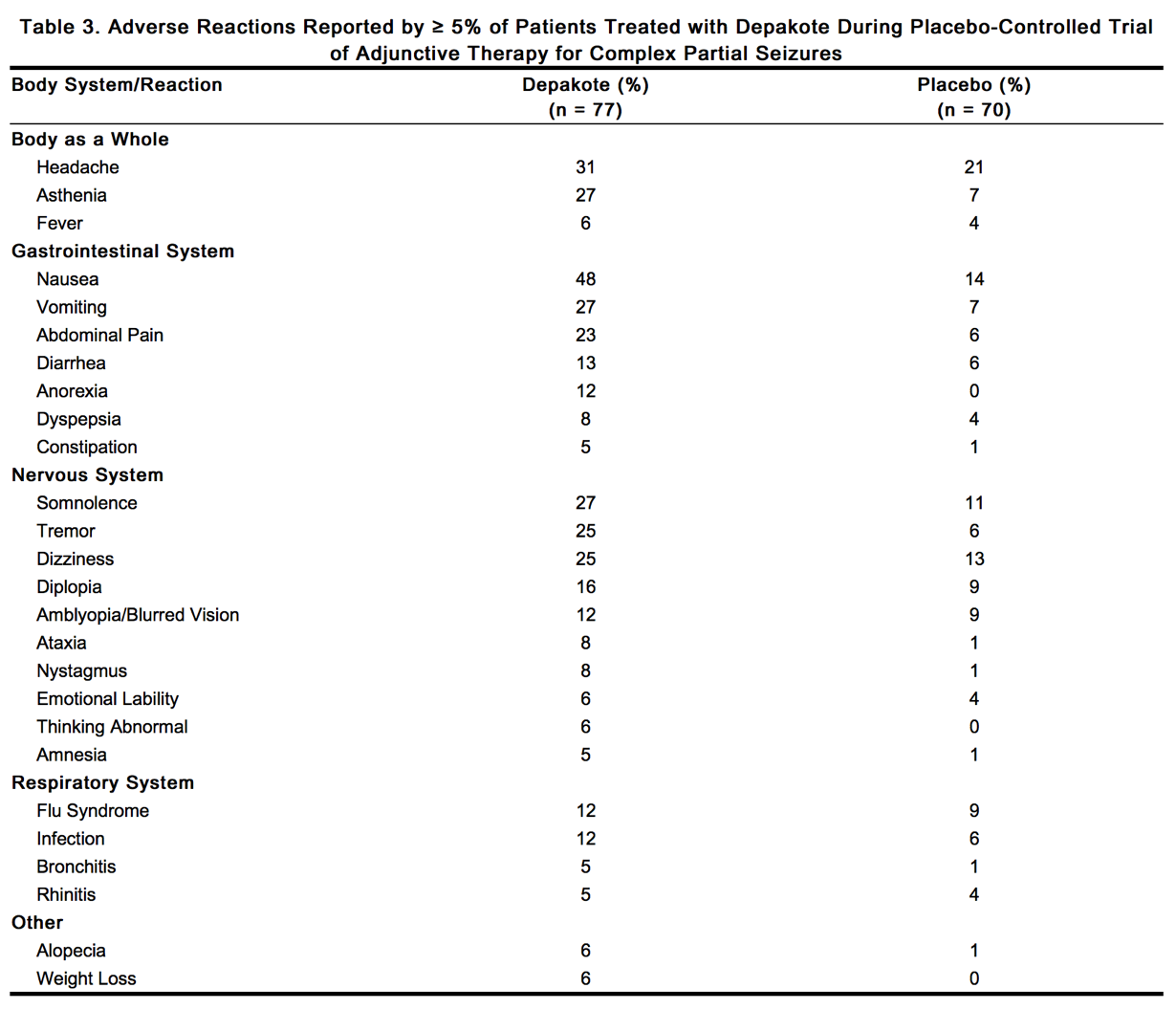 |
Table 4 lists treatment-emergent adverse reactions which were reported by ≥ 5% of patients in the high dose valproate group, and for which the incidence was greater than in the low dose group, in a controlled trial of Depakote monotherapy treatment of complex partial seizures. Since patients were being titrated off another antiepilepsy drug during the first portion of the trial, it is not possible, in many cases, to determine whether the following adverse reactions can be ascribed to Depakote alone, or the combination of valproate and other antiepilepsy drugs.
 |
The following additional adverse reactions were reported by greater than 1% but less than 5% of the 358 patients treated with valproate in the controlled trials of complex partial seizures:
- Body as a Whole: Back pain, chest pain, malaise.
- Cardiovascular System: Tachycardia, hypertension, palpitation.
- Digestive System: Increased appetite, flatulence, hematemesis, eructation, pancreatitis, periodontal abscess.
- Hemic and Lymphatic System: Petechia.
- Metabolic and Nutritional Disorders: SGOT increased, SGPT increased.
- Musculoskeletal System: Myalgia, twitching, arthralgia, leg cramps, myasthenia.
- Nervous System: Anxiety, confusion, abnormal gait, paresthesia, hypertonia, incoordination, abnormal dreams, personality disorder.
- Respiratory System: Sinusitis, cough increased, pneumonia, epistaxis.
- Skin and Appendages: Rash, pruritus, dry skin.
- Special Senses: Taste perversion, abnormal vision, deafness, otitis media.
- Urogenital System: Urinary incontinence, vaginitis, dysmenorrhea, amenorrhea, urinary frequency.
6.3 Migraine
Based on two placebo-controlled clinical trials and their long term extension, valproate was generally well tolerated with most adverse reactions rated as mild to moderate in severity. Of the 202 patients exposed to valproate in the placebo-controlled trials, 17% discontinued for intolerance. This is compared to a rate of 5% for the 81 placebo patients. Including the long term extension study, the adverse reactions reported as the primary reason for discontinuation by ≥ 1% of 248 valproate-treated patients were alopecia (6%), nausea and/or vomiting (5%), weight gain (2%), tremor (2%), somnolence (1%), elevated SGOT and/or SGPT (1%), and depression (1%).
Table 5 includes those adverse reactions reported for patients in the placebo-controlled trials where the incidence rate in the Depakote-treated group was greater than 5% and was greater than that for placebo patients.
 |
The following additional adverse reactions were reported by greater than 1% but not more than 5% of the 202 Depakote-treated patients in the controlled clinical trials:
- Body as a Whole: Chest pain, chills, face edema, fever and malaise.
- Cardiovascular System: Vasodilatation.
- Digestive System: Anorexia, constipation, dry mouth, flatulence, gastrointestinal disorder (unspecified), and stomatitis.
- Hemic and Lymphatic System: Ecchymosis.
- Metabolic and Nutritional Disorders: Peripheral edema, SGOT increase, and SGPT increase.
- Musculoskeletal System: Leg cramps and myalgia.
- Nervous System: Abnormal dreams, amnesia, confusion, depression, emotional lability, insomnia, nervousness, paresthesia, speech disorder, thinking abnormalities, and vertigo.
- Respiratory System: Cough increased, dyspnea, rhinitis, and sinusitis.
- Skin and Appendages: Pruritus and rash.
- Special Senses: Conjunctivitis, ear disorder, taste perversion, and tinnitus.
- Urogenital System: Cystitis, metrorrhagia, and vaginal hemorrhage.
6.4 Other Patient Populations
Adverse reactions that have been reported with all dosage forms of valproate from epilepsy trials, spontaneous reports, and other sources are listed below by body system.
Gastrointestinal
The most commonly reported side effects at the initiation of therapy are nausea, vomiting, and indigestion. These effects are usually transient and rarely require discontinuation of therapy. Diarrhea, abdominal cramps, and constipation have been reported. Both anorexia with some weight loss and increased appetite with weight gain have also been reported. The administration of delayed-release divalproex sodium may result in reduction of gastrointestinal side effects in some patients.
CNS Effects
Sedative effects have occurred in patients receiving valproate alone but occur most often in patients receiving combination therapy. Sedation usually abates upon reduction of other antiepileptic medication. Tremor (may be dose-related), hallucinations, ataxia, headache, nystagmus, diplopia, asterixis, "spots before eyes", dysarthria, dizziness, confusion, hypesthesia, vertigo, incoordination, and parkinsonism have been reported with the use of valproate. Rare cases of coma have occurred in patients receiving valproate alone or in conjunction with phenobarbital. In rare instances encephalopathy with or without fever has developed shortly after the introduction of valproate monotherapy without evidence of hepatic dysfunction or inappropriately high plasma valproate levels. Although recovery has been described following drug withdrawal, there have been fatalities in patients with hyperammonemic encephalopathy, particularly in patients with underlying urea cycle disorders [see Warnings and Precautions (5.6)].
There have been postmarketing reports of reversible and irreversible cerebral and cerebellar atrophy temporally associated with the use of valproate products. In some cases the patients recovered with permanent sequelae [see Warnings and Precautions (5.7)]. Cerebral atrophy has been reported in children exposed to valproate in utero [see Use in Specific Populations (8.1)].
Dermatologic
Transient hair loss, skin rash, photosensitivity, generalized pruritus, erythema multiforme, and Stevens-Johnson syndrome. Rare cases of toxic epidermal necrolysis have been reported including a fatal case in a 6 month old infant taking valproate and several other concomitant medications. An additional case of toxic epidermal necrosis resulting in death was reported in a 35 year old patient with AIDS taking several concomitant medications and with a history of multiple cutaneous drug reactions. Serious skin reactions have been reported with concomitant administration of lamotrigine and valproate [see Drug Interactions (7.2)].
Psychiatric
- Emotional upset, depression, psychosis, aggression, hyperactivity, hostility, and behavioral deterioration.
- Musculoskeletal
- Weakness.
- Hematologic
Thrombocytopenia and inhibition of the secondary phase of platelet aggregation may be reflected in altered bleeding time, petechiae, bruising, hematoma formation, epistaxis, and frank hemorrhage [see Warnings and Precautions (5.9) and Drug Interactions (7)]. Relative lymphocytosis, macrocytosis, hypofibrinogenemia, leucopenia, eosinophilia, anemia including macrocytic with or without folate deficiency, bone marrow suppression, pancytopenia, aplastic anemia, agranulocytosis, and acute intermittent porphyria.
Hepatic
Minor elevations of transaminases (e.g., SGOT and SGPT) and LDH are frequent and appear to be dose-related. Occasionally, laboratory test results include increases in serum bilirubin and abnormal changes in other liver function tests. These results may reflect potentially serious hepatotoxicity [see Warnings and Precautions (5.1)].
Endocrine
Irregular menses, secondary amenorrhea, breast enlargement, galactorrhea, and parotid gland swelling. Abnormal thyroid function tests [see Warnings and Precautions (5.17)].
There have been rare spontaneous reports of polycystic ovary disease. A cause and effect relationship has not been established.
Pancreatic
Acute pancreatitis including fatalities [see Warnings and Precautions (5.5)].
Metabolic
Hyperammonemia [see Warnings and Precautions (5.10,5.11)], hyponatremia, and inappropriate ADH secretion.
There have been rare reports of Fanconi's syndrome occurring chiefly in children.
Decreased carnitine concentrations have been reported although the clinical relevance is undetermined.
Hyperglycinemia has occurred and was associated with a fatal outcome in a patient with preexistent nonketotic hyperglycinemia.
Genitourinary
Enuresis and urinary tract infection.
Special Senses
Hearing loss, either reversible or irreversible, has been reported; however, a cause and effect relationship has not been established. Ear pain has also been reported.
Other
Allergic reaction, anaphylaxis, edema of the extremities, lupus erythematosus, bone pain, cough increased, pneumonia, otitis media, bradycardia, cutaneous vasculitis, fever, and hypothermia [see Warnings and Precautions (5.12)].
There have been reports of developmental delay, autism and/or autism spectrum disorder in the offspring of women exposed to valproate during pregnancy.[1]
References
Adapted from the FDA Package Insert.