Dipyridamole (tablet)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Adeel Jamil, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Dipyridamole (tablet) is a platelet aggregation inhibitor that is FDA approved for the treatment of postoperative thromboembolic complications of cardiac valve replacement. Common adverse reactions include chest pain, electrocardiogram abnormalities, flushing, rash, abdominal discomfort, dizziness, headache, and dyspnea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Adjunctive Use in Prophylaxis of Thromboembolism after Cardiac Valve Replacement
- Dosing Information
- The recommended dose is 75-100 mg four times daily as an adjunct to the usual warfarin therapy.
- Please note that aspirin is not to be administered concomitantly with coumarin anticoagulants.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Prophylaxis of Cerebrovascular Accident
- Developed by: ACCP
- Class of Recommendation: Class IIb
- Strength of Evidence: Category B
- Dosing Information
Non–Guideline-Supported Use
Peripheral Arterial Occlusive Disease
- Dosing Information
- 75 mg daily and aspirin 330 mg daily for 2 years[3]
Prophylaxis of Vascular Graft Occlusion
- Dosing Information
- 100 mg 4 times per day before the operation followed by DIPYRIDAMOLE 75 mg 3 times per day plus ASPIRIN 325 mg 3 times per day after the operation[4]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and effectiveness in the pediatric population below the age of 12 years have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Dipyridamole (tablet) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Dipyridamole (tablet) in pediatric patients.
Contraindications
- Hypersensitivity to dipyridamole and any of the other components.
Warnings
Precautions
- General
- Coronary Artery Disease: Dipyridamole has a vasodilatory effect and should be used with caution in patients with severe coronary artery disease (e.g., unstable angina or recently sustained myocardial infarction). Chest pain may be aggravated in patients with underlying coronary artery disease who are receiving dipyridamole.
- Hepatic Insufficiency: Elevations of hepatic enzymes and hepatic failure have been reported in association with dipyridamole administration.
- Hypotension: Dipyridamole should be used with caution in patients with hypotension since it can produce peripheral vasodilation.
- Laboratory Tests
- Dipyridamole has been associated with elevated hepatic enzymes.
Adverse Reactions
Clinical Trials Experience
- Adverse reactions at therapeutic doses are usually minimal and transient. On long-term use of dipyridamole USP tablets initial side effects usually disappear. The following reactions in Table 1 were reported in two heart valve replacement trials comparing dipyridamole USP tablets and warfarin therapy to either warfarin alone or warfarin and placebo:
- Other reactions from uncontrolled studies include diarrhea, vomiting, flushing and pruritus. In addition, angina pectoris has been reported rarely and there have been rare reports of liver dysfunction. On those uncommon occasions when adverse reactions have been persistent or intolerable, they have ceased on withdrawal of the medication.
- When dipyridamole USP tablets were administered concomitantly with warfarin, bleeding was no greater in frequency or severity than that observed when warfarin was administered alone. In rare cases, increased bleeding during or after surgery has been observed. In post-marketing reporting experience, there have been rare reports of hypersensitivity reactions (such as rash, urticaria, severe bronchospasm, and angioedema), larynx edema, fatigue, malaise, myalgia, arthritis, nausea, dyspepsia, paresthesia, hepatitis, thrombocytopenia, alopecia, cholelithiasis, hypotension, palpitation, and tachycardia.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Dipyridamole (tablet) in the drug label.
Drug Interactions
- No pharmacokinetic drug-drug interaction studies were conducted with dipyridamole USP Tablets. The following information was obtained from the literature.
- Adenosine: Dipyridamole has been reported to increase the plasma levels and cardiovascular effects of adenosine. Adjustment of adenosine dosage may be necessary.
- Cholinesterase Inhibitors: Dipyridamole may counteract the anticholinesterase effect of cholinesterase inhibitors, thereby potentially aggravating myasthenia gravis.
Use in Specific Populations
Pregnancy
- Pregnancy Category B
- Reproduction studies have been performed in mice, rabbits and rats at oral dipyridamole doses of up to 125 mg/kg, 40 mg/kg and 1000 mg/kg, respectively (about 1 ½ , 2 and 25 times the maximum recommended daily human oral dose, respectively, on a mg/m2 basis) and have revealed no evidence of harm to the fetus due to dipyridamole. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, dipyridamole USP should be used during pregnancy only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Dipyridamole (tablet) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Dipyridamole (tablet) during labor and delivery.
Nursing Mothers
- As dipyridamole is excreted in human milk, caution should be exercised when dipyridamole USP tablets are administered to a nursing woman.
Pediatric Use
- Safety and effectiveness in the pediatric population below the age of 12 years have not been established.
Geriatic Use
There is no FDA guidance on the use of Dipyridamole (tablet) with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Dipyridamole (tablet) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Dipyridamole (tablet) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Dipyridamole (tablet) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Dipyridamole (tablet) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Dipyridamole (tablet) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Dipyridamole (tablet) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Dipyridamole (tablet) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Dipyridamole (tablet) in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- In case of real or suspected overdose, seek medical attention or contact a Poison Control Center immediately. Careful medical management is essential. Based upon the known hemodynamic effects of dipyridamole, symptoms such as warm feeling, flushes, sweating, restlessness, feeling of weakness and dizziness may occur. A drop in blood pressure and tachycardia might also be observed.
Management
- Symptomatic treatment is recommended, possibly including a vasopressor drug. Gastric lavage should be considered. Administration of xanthine derivatives (e.g., aminophylline) may reverse the hemodynamic effects of dipyridamole overdose. Since dipyridamole is highly protein bound, dialysis is not likely to be of benefit.
Chronic Overdose
There is limited information regarding Chronic Overdose of Dipyridamole (tablet) in the drug label.
Pharmacology
| |
| |
Dipyridamole (tablet)
| |
| Systematic (IUPAC) name | |
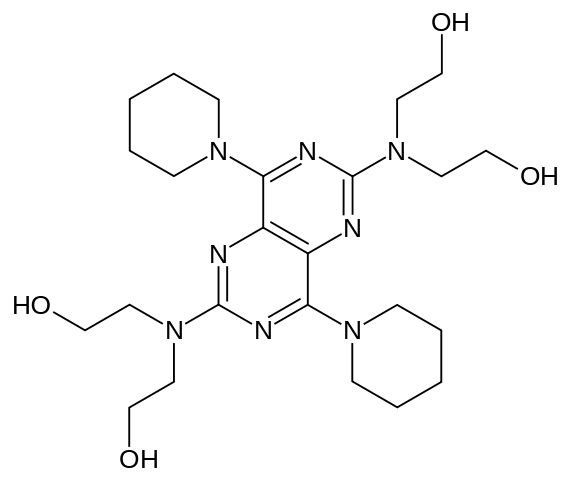
| 2,2',2'',2'''-(4,8-di(piperidin-1-yl)pyrimido[5,4-d]pyrimidine-2,6-diyl)bis(azanetriyl)tetraethanol | |
| Identifiers | |
| CAS number | |
| ATC code | B01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 504.626 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | 99% |
| Metabolism | Hepatic |
| Half life | Alpha (40 mins), Beta (10 Hours) |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
B |
| Legal status |
POM(UK) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | oral, IV |
Mechanism of Action
- Dipyridamole inhibits the uptake of adenosine into platelets, endothelial cells and erythrocytes in vitro and in vivo; the inhibition occurs in a dose-dependent manner at therapeutic concentrations (0.5-1.9 µg/mL). This inhibition results in an increase in local concentrations of adenosine which acts on the platelet A2-receptor thereby stimulating platelet adenylate cyclase and increasing platelet cyclic-3',5'-adenosine monophosphate (cAMP) levels. Via this mechanism, platelet aggregation is inhibited in response to various stimuli such as platelet activating factor (PAF), collagen and adenosine diphosphate (ADP).
- Dipyridamole inhibits phosphodiesterase (PDE) in various tissues. While the inhibition of cAMP-PDE is weak, therapeutic levels of dipyridamole inhibit cyclic-3',5'-guanosine monophosphate-PDE (cGMP-PDE), thereby augmenting the increase in cGMP produced by EDRF (endothelium-derived relaxing factor, now identified as nitric oxide).
Hemodynamics
- In dogs intraduodenal doses of dipyridamole of 0.5 to 4.0 mg/kg produced dose-related decreases in systemic and coronary vascular resistance leading to decreases in systemic blood pressure and increases in coronary blood flow. Onset of action was in about 24 minutes and effects persisted for about 3 hours.
- Similar effects were observed following IV dipyridamole USP in doses ranging from 0.025 to 2.0 mg/kg.
- In man the same qualitative hemodynamic effects have been observed. However, acute intravenous administration of dipyridamole USP may worsen regional myocardial perfusion distal to partial occlusion of coronary arteries.
Structure
- Dipyridamole USP is a platelet inhibitor chemically described as 2,2',2",2"'-[(4,8-Dipiperidinopyrimido[5,4-d]pyrimidine-2,6-diyl)dinitrilo]-tetraethanol. It has the following structural formula:
- Dipyridamole is an odorless yellow crystalline powder, having a bitter taste. It is soluble in dilute acids, methanol and chloroform, and practically insoluble in water.
- Dipyridamole USP tablets for oral administration contain:
- Active Ingredient TABLETS 25 mg, 50 mg, and 75 mg: dipyridamole USP 25 mg, 50 mg and 75 mg, respectively.
- Inactive Ingredients TABLETS 25 mg, 50 mg, and 75 mg: corn starch, hydroxypropyl methylcellulose, lactose monohydrate, magnesium stearate, polyethylene glycol, povidone, and titanium dioxide.
Pharmacodynamics
- It is believed that platelet reactivity and interaction with prosthetic cardiac valve surfaces, resulting in abnormally shortened platelet survival time, is a significant factor in thromboembolic complications occurring in connection with prosthetic heart valve replacement.
- Dipyridamole USP tablets have been found to lengthen abnormally shortened platelet survival time in a dose-dependent manner.
- In three randomized controlled clinical trials involving 854 patients who had undergone surgical placement of a prosthetic heart valve, dipyridamole USP tablets, in combination with warfarin, decreased the incidence of postoperative thromboembolic events by 62 to 91 % compared to warfarin treatment alone. The incidence of thromboembolic events in patients receiving the combination of dipyridamole USP tablets and warfarin ranged from 1.2 to 1.8%. In three additional studies involving 392 patients taking dipyridamole USP tablets and coumarin-like anticoagulants, the incidence of thromboembolic events ranged from 2.3 to 6.9%.
- In these trials, the coumarin anticoagulant was begun between 24 hours and 4 days postoperatively, and the dipyridamole USP tablets were begun between 24 hours and 10 days postoperatively. The length of follow-up in these trials varied from 1 to 2 years.
- Dipyridamole USP tablets do not influence prothrombin time or activity measurements when administered with warfarin.
Pharmacokinetics
- Following an oral dose of dipyridamole USP tablets, the average time to peak concentration is about 75 minutes. The decline in plasma concentration following a dose of Dipyridamole USP tablets fits a two-compartment model. The alpha half-life (the initial decline following peak concentration) is approximately 40 minutes. The beta half-life (the terminal decline in plasma concentration) is approximately 10 hours. Dipyridamole is highly bound to plasma proteins. It is metabolized in the liver where it is conjugated as a glucuronide and excreted with the bile.
Nonclinical Toxicology
- Carcinogenesis, Mutagenesis, Impairment of Fertility
- In studies in which dipyridamole was administered in the feed to mice (up to 111 weeks in males and females) and rats (up to 128 weeks in males and up to 142 weeks in females), there was no evidence of drug-related carcinogenesis. The highest dose administered in these studies (75 mg/kg/day) was, on a mg/m2 basis, about equivalent to the maximum recommended daily human oral dose (MRHD) in mice and about twice the MRHD in rats. Mutagenicity tests of dipyridamole with bacterial and mammalian cell systems were negative. There was no evidence of impaired fertility when dipyridamole was administered to male and female rats at oral doses up to 500 mg/kg/day (about 12 times the MRHD on a mg/m2 basis). A significant reduction in number of corpora lutea with consequent reduction in implantations and live fetuses was, however, observed at 1250 mg/kg (more than 30 times the MRHD on a mg/m2 basis).
Clinical Studies
There is limited information regarding Clinical Studies of Dipyridamole (tablet) in the drug label.
How Supplied
- Dipyridamole tablets, USP, are available as round, white, film-coated tablets of 25 mg, 50 mg, and 75 mg coded 81/SL, 82/SL, and 83/SL, respectively.
- They are available in unit dose box of 100 tablets as indicated below:
- 25 mg Tablets (NDC 0904-1086-61)
- 50 mg Tablets (NDC 0904-1087-61)
- 75 mg Tablets (NDC 0904-1088-61)
- STORE AT 25°C (77°F); EXCURSIONS PERMITTED TO 15-30°C (59°-86°F) [SEE USP CONTROLLED ROOM TEMPERATURE]. KEEP OUT OF REACH OF CHILDREN.
Storage
There is limited information regarding Dipyridamole (tablet) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Dipyridamole (tablet) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Dipyridamole (tablet) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Dipyridamole (tablet) in the drug label.
Precautions with Alcohol
- Alcohol-Dipyridamole (tablet) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Persantine®[5]
Look-Alike Drug Names
- N/A[6]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Diener, H. C. (2001-04). "Cardiac safety in the European Stroke Prevention Study 2 (ESPS2)". International Journal of Clinical Practice. 55 (3): 162–163. ISSN 1368-5031. PMID 11351768. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ Diener, H. C. (1998-09). "Dipyridamole trials in stroke prevention". Neurology. 51 (3 Suppl 3): –17-19. ISSN 0028-3878. PMID 9744826. Check date values in:
|date=(help) - ↑ Hess, H. (1985-02-23). "Drug-induced inhibition of platelet function delays progression of peripheral occlusive arterial disease. A prospective double-blind arteriographically controlled trial". Lancet. 1 (8426): 415–419. ISSN 0140-6736. PMID 2857803. Unknown parameter
|coauthors=ignored (help) - ↑ Norcott, H. C. (1982-12-27). "Platelet inhibitory drugs: an in vivo method of evaluation in patients". Thrombosis and Haemostasis. 48 (3): 307–310. ISSN 0340-6245. PMID 6761890. Unknown parameter
|coauthors=ignored (help) - ↑ "DIPYRIDAMOLE tablet, film coated".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Dipyridamole (tablet)
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Dipyridamole (tablet) |Label Name=Dipyridamole04.png
}}
{{#subobject:
|Label Page=Dipyridamole (tablet) |Label Name=Dipyridamole05.png
}}

