Covalent bond
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Covalent bonds a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms, or between atoms and other covalent bonds. In short, attraction-to-repulsion stability that forms between atoms when they share electrons is known as covalent bonding.
Covalent bonding includes many kinds of interactions, including σ-bonding, π-bonding, metal-metal bonding, agostic interactions, and three-center two-electron bonds.[1][2] The term covalent bond dates from 1939.[3] The prefix co- means jointly, associated in action, partnered to a lesser degree, etc.; thus a "co-valent bond", essentially, means that the atoms share "valence", such as is discussed in valence bond theory. In the molecule H2, the hydrogen atoms share the two electrons via covalent bonding. Covalency is greatest between atoms of similar electronegativities. Thus, covalent bonding does not necessarily require the two atoms be of the same elements, only that they be of comparable electronegativity. Because covalent bonding entails sharing of electrons, it is necessarily delocalized. Furthermore, in contrast to electrostatic interactions ("ionic bonds") the strength of covalent bond depends on the angular relation between atoms in polyatomic molecules.
History
The term "covalence" in regard to bonding was first used in 1919 by Irving Langmuir in a Journal of American Chemical Society article entitled The Arrangement of Electrons in Atoms and Molecules:[4]
| “ | (p.926)… we shall denote by the term covalence the number of pairs of electrons which a given atom shares with its neighbors. | ” |
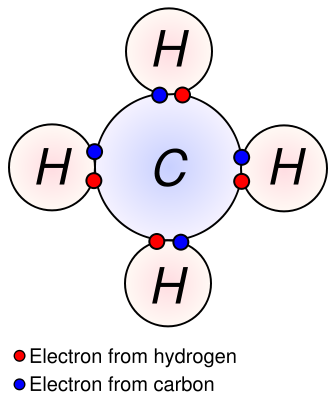
The idea of covalent bonding can be traced several years prior to 1920 to Gilbert N. Lewis, who in 1916 described the sharing of electron pairs between atoms. He introduced the so called Lewis notation or electron dot notation or The Lewis Dot Structure in which valence electrons (those in the outer shell) are represented as dots around the atomic symbols. Pairs of electrons located between atoms represent covalent bonds. Multiple pairs represent multiple bonds, such as double and triple bonds. Some examples of Electron Dot Notation are shown in the following figure. An alternative form, in which bond-forming electron pairs are represented as solid lines, is shown alongside.
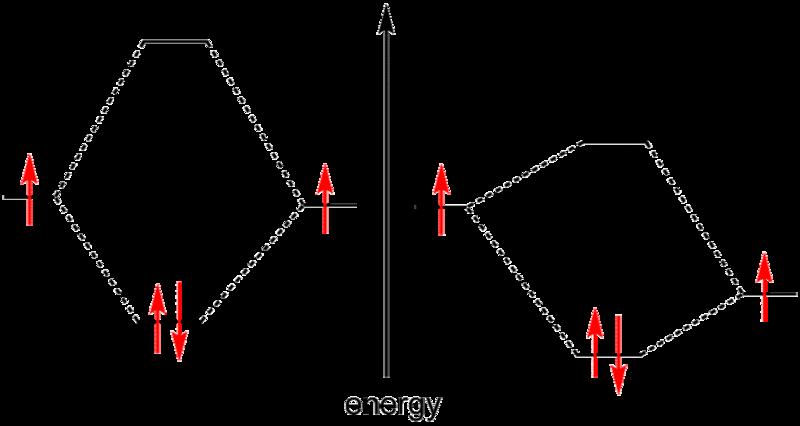
While the idea of shared electron pairs provides an effective qualitative picture of covalent bonding, quantum mechanics is needed to understand the nature of these bonds and predict the structures and properties of simple molecules. Walter Heitler and Fritz London are credited with the first successful quantum mechanical explanation of a chemical bond, specifically that of molecular hydrogen, in 1927.[5] Their work was based on the valence bond model, which assumes that a chemical bond is formed when there is good overlap between the atomic orbitals of participating atoms. These atomic orbitals are known to have specific angular relationships between each other, and thus the valence bond model can successfully predict the bond angles observed in simple molecules.
Bond order
Bond order is a number that indicates the number of pairs of electrons shared between atoms forming a covalent bond. The term is only applicable to diatomic molecules, but is used to describe bonds within polyatomic compounds as well.
- The most common type of covalent bond is the single bond, the sharing of only one pair of electrons between two atoms. It usually consists of one sigma bond. All bonds with more than one shared pair are called multiple bonds.
- Sharing two pairs is called a double bond. An example is in ethylene (between the carbon atoms). It usually consists of one sigma bond and one pi bond.
- Sharing three pairs is called a triple bond. An example is in hydrogen cyanide (between C and N). It usually consists of one sigma bond and two pi-bonds.
- Quadruple bonds are found in the transition metals. Molybdenum and rhenium are the elements most commonly observed with this bonding configuration. An example of a quadruple bond is also found in Di-tungsten tetra(hpp).
- Quintuple bonds have been found to exist in certain dichromium compounds.
- Sextuple bonds are found in diatomic molybdenum and tungsten.
Most bonding of course, is not localized, so the following classification, while powerful and pervasive, is of limited validity. Three center bonds do not conform readily to the above conventions.
Resonance
Many bonding situations can be described with more than one valid Lewis Dot Structure (for example, ozone, O3). In an LDS diagram of O3, the center atom will have a single bond with one atom and a double bond with the other. The LDS diagram cannot tell us which atom has the double bond; the first and second adjoining atoms have equal chances of having the double bond. These two possible structures are called resonance structures. In reality, the structure of ozone is a resonance hybrid between its two possible resonance structures. Instead of having one double bond and one single bond, there are actually two 1.5 bonds with approximately three electrons in each at all times.
A special resonance case is exhibited in aromatic rings of atoms (for example, benzene). Aromatic rings are composed of atoms arranged in a circle (held together by covalent bonds) that may alternate between single and double bonds according to their LDS. In actuality, the electrons tend to be disambiguously and evenly spaced within the ring. Electron sharing in aromatic structures is often represented with a ring inside the circle of atoms.
Lewis Dot Structures for molecules with resonance are shown by creating the dot structure for every possible form, placing brackets around each structure, and connecting the boxes with double-headed arrows.
Current theory
Today the valence bond model has been supplanted by the molecular orbital model. In this model, as atoms are brought together, the atomic orbitals interact to form molecular orbitals, which are linear sums and differences of the atomic orbitals. These molecular orbitals are a cross between the original atomic orbitals and generally extend between the two bonding atoms.
Using quantum mechanics it is possible to calculate the electronic structure, energy levels, bond angles, bond distances, dipole moments, and electromagnetic spectra of simple molecules with a high degree of accuracy. Bond distances and angles can be calculated as accurately as they can be measured (distances to a few pm and bond angles to a few degrees). For small molecules, calculations are sufficiently accurate to be useful for determining thermodynamic heats of formation and kinetic activation energy barriers.
References
- ↑ March, J. “Advanced Organic Chemistry” 4th Ed. J. Wiley and Sons, 1992: New York. ISBN 0-471-60180-2.
- ↑ G. L. Miessler and D. A. Tarr “Inorganic Chemistry” 3rd Ed, Pearson/Prentice Hall publisher, ISBN 0-13-035471-6.
- ↑ Merriam-Webster - Collegiate Dictionary (2000).
- ↑ Langmuir, I. (1919). J. Am. Chem. Soc.; 1919; 41; 868-934.
- ↑ W. Heitler and F. London, Zeitschrift für Physik, vol. 44, p. 455 (1927). English translation in H. Hettema, Quantum Chemistry, Classic Scientific Papers, World Scientific, Singapore (2000).
See also
- Metallic bonding
- Linear combination of atomic orbitals
- Hybridization
- Hydrogen bond
- Noncovalent bonding
- Disulfide bond
Additional Resources
- "Covalent bonding - Single bonds". chemguide. 2000.
- "Electron Sharing and Covalent Bonds". Department of Chemistry University of Oxford.
- "Chemical Bonds". Department of Physics and Astronomy, Georgia State University.
External links
Template:Organic chemistry Template:Chemical bonds
ar:رابطة تساهمية bs:Kovalentna veza bg:Ковалентна химична връзка ca:Enllaç covalent cs:Kovalentní vazba da:Kovalent binding de:Atombindung et:Kovalentne side el:Ομοιοπολικός δεσμός fa:پیوند کووالانسی gl:Enlace covalente ko:공유 결합 hr:Kovalentna veza it:Legame covalente he:קשר קוולנטי hu:Kovalens kötés mk:Ковалентна врска nl:Covalente binding no:Kovalent binding nn:Kovalent binding simple:Covalent bond sk:Kovalentná väzba sl:Kovalentna vez su:Beungkeut kovalén fi:Kovalenttinen sidos sv:Kovalent bindning th:พันธะโควาเลนต์ uk:Ковалентний зв'язок