Cefaclor microbiology
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Microbiology
In vitro tests demonstrate that the bactericidal action of the cephalosporins results from inhibition of cell-wall synthesis. Cefaclor has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section.
Aerobes, Gram-positive
- Staphylococci, including coagulase-positive,coagulase-negative, and penicillinase-producing strains
- Streptococcus pyogenes (group A β-hemolytic [[streptococci]])
Aerobes, Gram-negative
- Haemophilus influenzae, excluding β-lactamase negative ampicillin-resistant strains
- Klebsiella spp.
The following in vitro data are available, but their clinical significance is unknown.
Cefaclor exhibits in vitro minimal inhibitory concentrations (MICs) of ≤8 μg/mL against most (≥ 90%) strains of the following microorganisms; however, the safety and effectiveness of cefaclor in treating clinical infections due to these microorganisms have not been established in adequate and well-controlled clinical trials.
Aerobes, Gram-negative
- Citrobacter diversus
- Moraxella (Branhamella) catarrhalis
AnAerobes, Gram-positive
- Bacteroides spp. (excluding Bacteroides fragilis)
Note: Pseudomonas spp., Acinetobacter calcoaceticusand most strains of enterococci (Enterococcus faecalis, group D streptococci), Enterobacter spp.,indole-positive Proteus, Morganella morganii (formerly Proteus morganii), Providencia rettgeri(formerly Proteus rettgeri), and Serratia spp. Are resistant to cefaclor. When tested by in vitro methods, Staphylococci exhibit cross-resistance between cefaclor and methicillin-type antibiotics.
Susceptibility Testing
Dilution Techniques - Quantitative methods that are used to determine minimum inhibitory concentrations (MIC) provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure that has been recommended for use with cefaclor powder uses a standardized dilution method1(broth, agar, or microdilution). The MIC values obtained should be interpreted according to the following criteria:
 |
Note: β-lactamase-negative, ampicillin-resistant strains of H. influenzae should be considered resistant to cefaclor despite apparent in vitro susceptibility to this agent.
A report of "Susceptible" indicates that the pathogen is likely to be inhibited by usually achievable concentrations of the antimicrobial compound in blood. A report of "Intermediate" indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone that prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of "Resistant" indicates that usually achievable concentrations of the antimicrobial compound in the blood are unlikely to be inhibitory and that other therapy should be selected.
Standardized susceptibility test procedures require the use of laboratory control microorganisms. Standard cefaclor powder should provide the following MIC values:
 |
Dilution Techniques - Quantitative methods that require measurement of zone diameters provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure 2 that has been recommended for use with disks to test the susceptibility of microorganisms to cefaclor uses the 30-μg cefaclor disk. Interpretation involves correlation of the diameter obtained in the disk test with the MIC for cefaclor. Reports from the laboratory providing results of the standard single-disk susceptibility test with a 30-μg cefaclor disk should be interpreted according to the following criteria:
 |
Note: β-lactamase-negative, ampicillin-resistant strains of H. influenzae should be considered resistant to cefaclor despite apparent in vitro susceptibility to this agent.
Interpretation should be as stated above for results using dilution techniques.
As with standard dilution techniques, diffusion methods require the use of laboratory control microorganisms. The 30-μg cefaclor disk should provide the following zone diameters in these laboratory test quality control strains[1]:
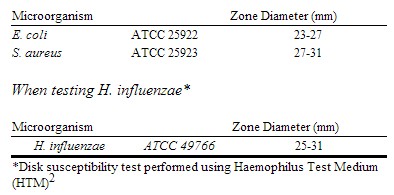 |
References
- ↑ publisher = "http://www.accessdata.fda.gov/drugsatfda_docs/nda/98/64081_Cefaclor_Approv.pdf" Check
|url=value (help). External link in|title=(help)
Adapted from the FDA Package Insert.