Baylis-Hillman reaction
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
The Baylis-Hillman reaction is an organic reaction of an aldehyde and an α,β-unsaturated electron-withdrawing group catalyzed by DABCO (1,4-diazabicyclo[2.2.2]octane) to give an allylic alcohol [2]. This reaction is also known as the Morita–Baylis–Hillman reaction or MBH reaction [3]. It is named for the Japanese chemist Ken-ichi Morita, and the German chemists Anthony B. Baylis and Melville E. D. Hillman.
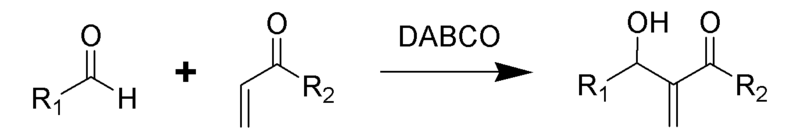
In addition to DABCO, additional nucleophilic amines such as DMAP and DBU have been found to successfully catalyze this reaction.
Reaction mechanism
The nucleophilic addition of DABCO 2 onto the α,β-unsaturated ketone 1 gives a charged intermediate 3, which will add to the electrophilic aldehyde producing the keto-alcohol 4. Elimination of the DABCO gives the desired allylic alcohol 5.
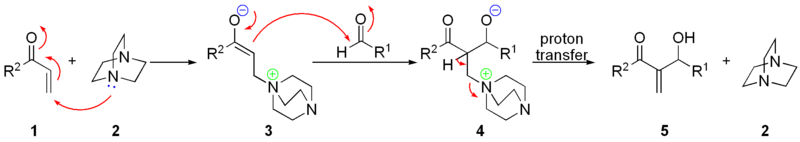
An alternative mechanism, based on extensive rate data, has been proposed for some aldehydes.[4] [5]
A related reaction actually predating the Baylis-Hillman reaction utilising phosphines and not DABCO is the lesser known Rauhut-Currier reaction.
Scope
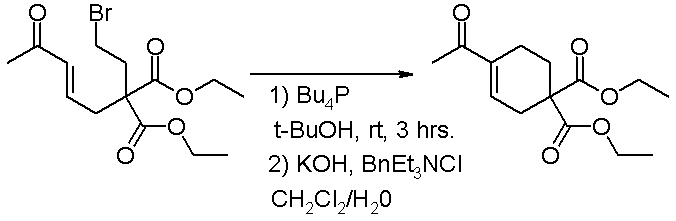
The MBH reaction in general is any reaction of electron deficient alkenes and sp2 hybridized carbon electrophiles such as aldehydes, ketones and aldimines catalyzed by a nucleophile. Under special reaction conditions the reaction is also found to extend to alkyl halides as the electrophilic reagent [6]. In this variation amine nucleophiles are unsuitable and trialkyl phosphines are used instead. Under the given reaction conditions these phosphines do not react directly with the alkyl halide. The added base in the second step of this reaction promotes the elimination reaction to the enone.
In the aza-Baylis-Hillman reaction the electrophile is an imine.[7]
Limitations
The MBH reaction of phenyl vinyl ketone with benzaldehyde and DABCO in DMF is not limited to the monoadduct because the MBH adduct reacts with a second molecule of phenyl vinyl ketone in a nucleophilic conjugate addition [8].
References
- ^ Baylis, A. B.; Hillman, M. E. D. German Patent 2155113, 1972.
- ^ K. Morita, Z. Suzuki and H. Hirose, Bull. Chem. Soc. Jpn.,1968, 41, 2815.
- ^ Price, K. E.; Broadwater, S. J.; Jung, H. M.; McQuade, D. T.; Org. Lett., 2005, 7(1), 147-150.
- ^ Drewes, S. E.; Roos, G. H. P.; Tetrahedron 1988, 44, 4653-4670.
- ^ Unprecedented reactivity in the Morita–Baylis–Hillman reaction; intramolecular -alkylation of enones using saturated alkyl halides Marie E. Krafft, Kimberly A. Seibert, Thomas F. N. Haxell and Chitaru Hirosawa Chemical Communications, 2005, (46), 5772 - 5774 DOI: 10.1039/b512665g Abstract
- ^ Enantioselective aza-Baylis-Hillman Reaction Vasco D.B. Bonifacio, Org. Chem. Highlights, 2006, Full Article
- ^ Different Reaction Patterns in the Baylis-Hillman Reaction of Aryl Aldehydes with Phenyl Vinyl Ketone, Phenyl Acrylate and Phenyl Thioacrylate Min Shi, Chao-Qun Li and Jian-Kang Jiang Molecules 2002, 7, 721-733 Full Article