Anti-neutrophil cytoplasmic antibody
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Anti-neutrophil cytoplasmic antibodies (ANCAs) are a group of mainly IgG antibodies against antigens in the cytoplasm of neutrophil granulocytes (the most common type of white blood cell) and monocytes. They are detected as a blood test in a number of autoimmune disorders, but are particularly associated with systemic vasculitis, so called ANCA-associated vasculitides.
Types
ANCA were originally shown to divide into two main classes, c-ANCA and p-ANCA, based on the pattern of staining on ethanol-fixed neutrophils and the main target antigen. ANCA titres are generally measured using ELISA and indirect immunofluorescence.[1]
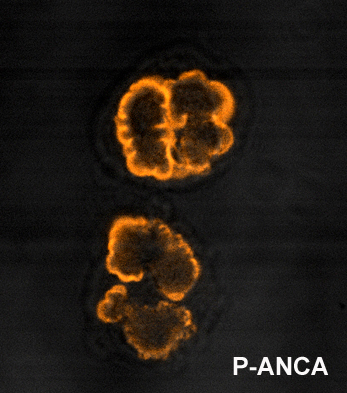
p-ANCA
p-ANCA, or perinuclear-staining antineutrophil cytoplasmic antibodies, show a perinuclear staining pattern. This pattern occurs because during ethanol fixation some antigen targets artifactually localize around the nucleus. Antibody staining therefore results in fluorescence of the region around the nucleus. By far the most common p-ANCA target is myeloperoxidase (MPO), a neutrophil granule protein whose primary role in normal metabolic processes is generation of oxygen radicals. ANCA will less commonly form against alternative antigens that may also result in a p-ANCA pattern. These include lactoferrin; elastase; and cathepsin G. p-ANCA is fairly sensitive, but not specific for ulcerative colitis, so not useful as a sole diagnostic test.[2]
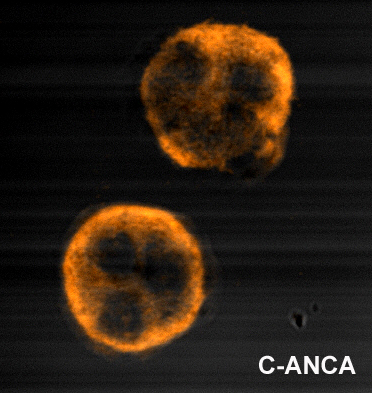
c-ANCA
c-ANCAs, or cytoplasmic-staining antineutrophil cytoplasmic antibodies, show a diffusely granular, cytoplasmic staining pattern. This pattern results from binding of ANCA to antigen targets throughout the neutrophil cytoplasm, the most common protein target being proteinase 3 (PR3). PR3 is the most common antigen target of ANCA in patients with Wegener's granulomatosis. Other antigens may also occasionally result in a c-ANCA pattern.
Other
ANCA that develop against antigens other than MPO or PR3 will occasionally result in patchy staining when visualized by immunofluorescence. This pattern is commonly called the 'snowdrift' pattern, and most commonly occurs in patients with non-vasculitic diseases that are associated with ANCA formation.
Development of ANCA
It is poorly understood how ANCA are developed, although several hypotheses have been suggested. There is probably a genetic contribution, particularly in genes controlling the level of immune response – although genetic susceptibility is likely to be linked to an environmental factor, some possible factors including vaccination or exposure to silicates. Two possible mechanisms of ANCA development are postulated, although neither of these theories answers the question of how the different ANCA specificities are developed, and there is much research still being undertaken on the development of ANCA.[3]
Theory of molecular mimicry
Microbial superantigens are molecules expressed by bacteria and other microorganisms that have the power to stimulate a strong immune response by activation of T-cells. These molecules generally have regions that resemble self-antigens – this is the theory of molecular mimicry. Staphylococcal and streptococcal superantigens have been characterised in autoimmune diseases – the classical example in post group A streptococcal rheumatic heart disease, where there is similarity between M proteins of Streptococcus pyogenes to cardiac myosin and laminin. It has also been shown that up to 70% of patients with Wegener's granulomatosis are chronic nasal carriers of Staphylococcus aureus, with carriers having an eight times increased risk of relapse.[3]
Theory of defective apoptosis
Neutrophil apoptosis, or programmed cell death, is vital in controlling the duration of the early inflammatory response, thus restricting damage to tissues by the neutrophils. ANCA may be developed either via ineffective apoptosis or ineffective removal of apoptotic cell fragments, leading to the exposure of the immune system to molecules normally sequestered inside the cells. This theory solves the paradox that the antigenic targets of ANCA are generally located intracellularly so therefore how is it possible for antibodies to be raised against them.[3]
Role in disease
There are three primary diseases that are consistently associated with ANCA: Wegener's granulomatosis, microscopic polyangiitis, and glomerulonephritis. The antibodies are assumed to be involved in the generation and/or progression of lesions and clinical signs.
Classically, c-ANCA is associated with Wegener’s granulomatosis; p-ANCA is associated with microscopic polyangiitis and focal necrotising and crescentic glomerulonephritis. However, in recent years ANCA targeted against other autoantigens have been identified.[4]
Patients with a number of other diseases, such as ulcerative colitis and ankylosing spondylitis, will commonly have ANCA as well. However in these cases there is no assocatied vasculitis, and the ANCA are thought to be incidental or epiphenomena rather than part of the disease itself. Churg-Strauss syndrome is associated with p-ANCA directed against MPO.[5]
It is unclear what the rôle of ANCA may be in these diseases – they may be markers of disease or may play some part in the pathogenic process. It has been shown that in Wegener's granulomatosis there is positive correlation between ANCA titre and disease activity and in vitro studies have shown that ANCA cause activation of primed neutrophils and react with endothelial cells expressing PR3.[3] ANCA may act by causing release of lytic enzymes from the white blood cells,[6] causing inflammation of the blood vessel wall (vasculitis). ANCA associated vasculitides usually present with features of a small-vessel vasculitis.
| cANCA | pANCA | |
|---|---|---|
| Staining pattern | Cytoplasmic | Perinuclear |
| Most common antigen | Proteinase 3 (PR3) | Myeloperoxidase (MPO) |
| Differential Diagnosis |
|
|
History
ANCAs were originally described in Davies et al in 1982 in segmental necrotising glomerulonephritis,[7] and by van der Woude et al in 1985 in Wegener's.[8] The Second International ANCA Workshop, held in The Netherlands in May 1989, fixed the nomenclature on perinuclear vs. cytoplasmic patterns, and the antigens MPO and PR3 were discovered in 1988 and 1989, respectively.[9]
Differential Diagnosis
- Churg-Strauss Vasculitis
- Crescentic glomerulonephritis
- Henoch-Schoenlein Purpura
- Inflammatory Bowel Disease
- Microscopic polyangitis
- Polyarteritis nodosa
- Primary sclerosing cholangitis
- Systemic Lupus Erythematosus
- Temporal Arteritis
- Wegener's Granulomatosis [10] [11]
References
- ↑ Radice A & Sinico RA. Antineutrophil cytoplasmic antibodies (ANCA). Autoimmunity 2005;38(1):93-103. PMID 15804710
- ↑ Shepherd B; et al. (2005). "Inflammatory Bowel Disease: Diagnostic and Treatment Options". Hospital Physician: 11–19.
- ↑ 3.0 3.1 3.2 3.3 Reumaux D, Duthilleul P, Roos D. Pathogenesis of diseases associated with antineutrophil cytoplasm autoantibodies. Hum Immunol 2004;65(1):1-12. PMID 14700590.
- ↑ Kain R, Matsui K, Exner M et al. A novel class of autoantigens of anti-neutrophil cytoplasmic antibodies in necrotizing and crescentic glomerulonephritis: the lysosomal membrane glycoprotein h-lamp-2 in neutrophil granulocytes and a related membrane protein in glomerular endothelial cells. J Exp Med 1995;181(2):585-597. PMID 7836914
- ↑ Seo P & Stone J. The Antineutrophil Cytoplasmic Antibody-Associated Vasculitides. Am J Med 2004;117:39-50. PMID 15210387
- ↑ Falk RJ, Terrell RS, Charles LA, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci U S A 1990;87:4115-4119. PMID 2161532.
- ↑ Davies DJ, Moran JE, Niall JF, Ryan GB. Segmental necrotising glomerulonephritis with antineutrophil antibody: possible arbovirus aetiology. Brit Med J 1982;285:606. PMID 6297657.
- ↑ van der Woude FJ, Rasmussen N, Lobatto S et al. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener's granulomatosis. Lancet 1985;1(8426):425-9. PMID 2857806.
- ↑ Jennette JC, Hoidal JR, Falk RJ. Specificity of anti-neutrophil cytoplasmic autoantibodies for proteinase 3. Blood 1990;75:2263-4. PDF (2 MB). PMID 2189509.
- ↑ Sailer, Christian, Wasner, Susanne. Differential Diagnosis Pocket. Hermosa Beach, CA: Borm Bruckmeir Publishing LLC, 2002:77 ISBN 1591032016
- ↑ Kahan, Scott, Smith, Ellen G. In A Page: Signs and Symptoms. Malden, Massachusetts: Blackwell Publishing, 2004:68 ISBN 140510368X
Links
- images of pANCA and cANCA
- fluorescence images of ANCA
- Anti-Neutrophil+Cytoplasmic+Antibody at the US National Library of Medicine Medical Subject Headings (MeSH)