Ancylostomiasis pathophysiology
|
Ancylostomiasis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Ancylostomiasis pathophysiology On the Web |
|
American Roentgen Ray Society Images of Ancylostomiasis pathophysiology |
|
Risk calculators and risk factors for Ancylostomiasis pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kalpana Giri, MBBS[2]
Overview
Ancylostomiasis is a hookworm infection, soil-transmitted helminths (STH) occurs predominantly in countries with low socioeconomic status located in tropical and subtropical areas of the world. The life cycle of hookworm include: human hookworm and zoonotic hookworm. Mature females released eggs in the host’s small intestine and these eggs are passed in the feces. Under appropriate conditions, each eggs hatch in soil, and develops into an infective filariform (L3) stage larva. It enter the body either through a skin or oral ingestion then enters the bloodstream, and reach the lungs and migrate across the alveoli. Then they ascend from the bronchial tree to the pharynx and reach the small intestine where they mount into fourth-stage larvae and mature into blood-feeding adults male or female.
Pathophysiology
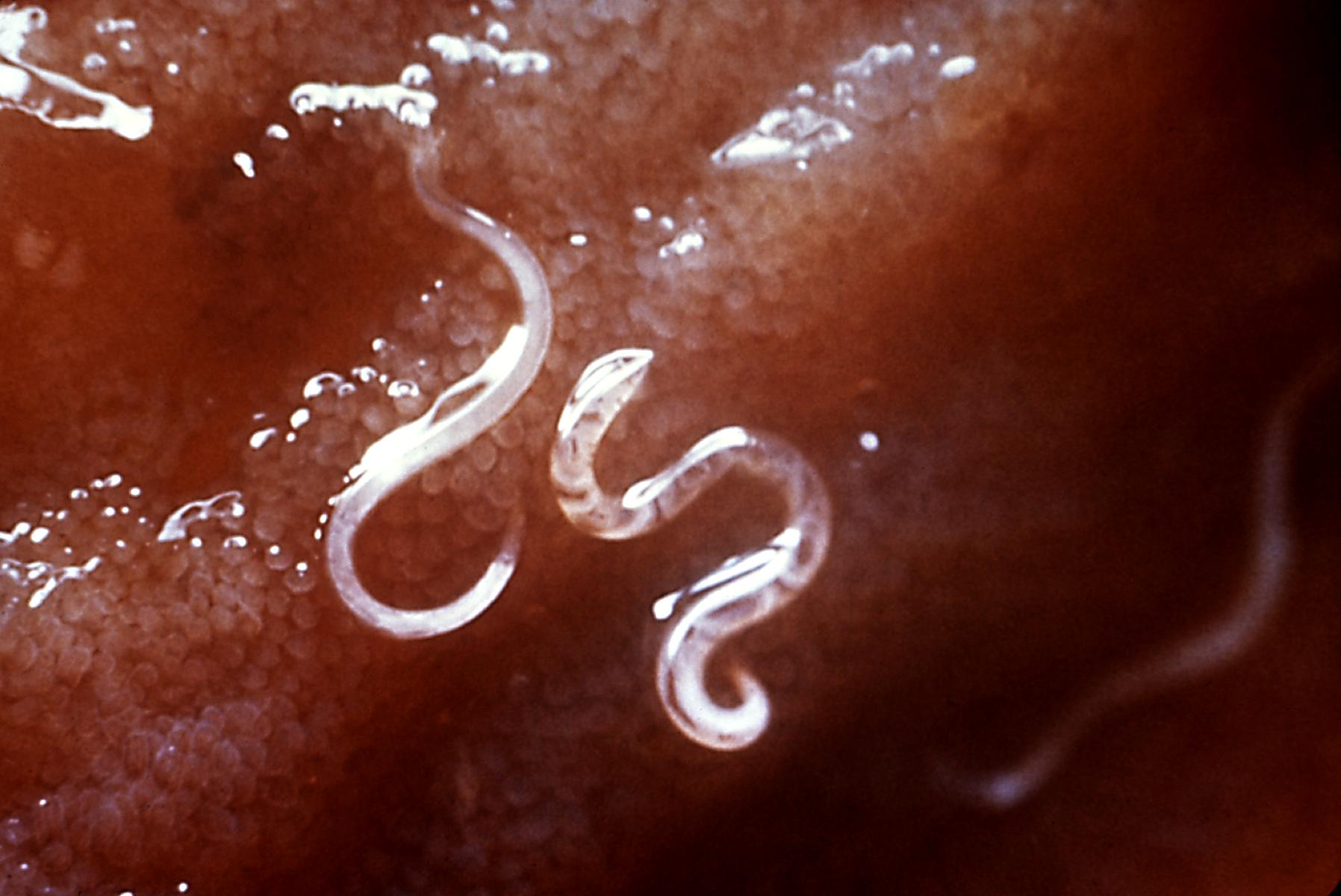
Ancylostomiasis is a hookworm infection, soil-transmitted helminths (STH) also known as miner's anaemia, tunnel disease, brickmaker's anaemia occurs predominantly in countries with low socioeconomic status located in tropical and subtropical areas of the world.[1][2] The external surface of Helminth comprises key molecules excretory/secretory (ES) products. Hookworm ES products contain a large range of structurally and functionally distinct molecules, mostly proteins, and also lipids, and carbohydrates. They are secreted from the oral orifice or outer surfaces of the parasite representing the boundary between the helminth parasites and their hosts. These molecules react with host proteins and are essential for the penetration of the host, tissue migration, nourishment, reproduction, and evasion of host immunity. These molecules also have major functions in the development and survival of parasites. By inhibiting the inflammatory reaction, encouraging effector cells apoptosis, and skewing the immune reaction phenotype, these molecules help the parasite to survive and evade the host immunological response. The biological role and molecular nature of hookworm ES products are still unclear though the intensive study has been done for many years.[3]
Hookworm life cycle
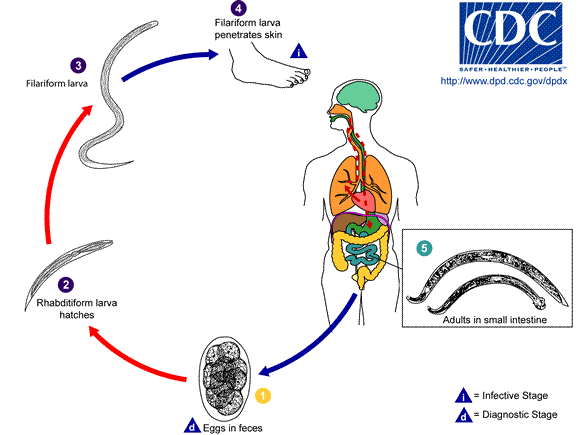
All species of hookworm have no intermediate host, they have a direct life cycle. Mature females released eggs in the host’s small intestine and these eggs are passed in the feces, where they hatch first stage rhabditiform larva (L1) within several days. The L1 feeds on soil microbes and molts to the L2 stage, and under appropriate conditions, each eggs hatch in warm, moist, sandy soil, or in feces and develops into an infective filariform (L3) stage larva. The infective-stage larvae (L3) enter the body either through a cutaneous route or by direct oral ingestion.
Human Hookworm
Some species of human hookworm such as Ancylostoma and Necator Americanus may cause a local pruritic dermatitis, also called ground itch at the site of penetration whereas the ancylostoma species can also enter the body orally.[4] The infective larvae (L3) migrate through the dermis, enters the bloodstream, and reach the lungs within 10 days. Once they reach the lungs, they migrate across the alveoli and breaks into alveoli leads to mild and usually asymptomatic alveolitis with eosinophilia. Then they ascend from the bronchial tree to the pharynx, during which the host may develop a cough, sore throat, fever. They coughed up, swallowed, and reach the small intestine where they mount into fourth-stage larvae and mature into blood-feeding adults male or female.[5] The adult worms attached to the mucosal layer of the small intestine, using their buccal capsule leads to arterioles and venules ruptures along with the luminal surface of the intestine. These adult worms release hyaluronidase and other hydrolytic enzymes result in blood extravasation by degrading the intestinal mucosa and erosion of blood vessels.[6] Hookworms also secrete Ancylostoma ceylanicum anticoagulant peptide-1, which inhibits the blood coagulation in the attachment site and leads to blood loss from the intestine.[7] The adult worms colonize the intestinal tract and in 3-5 weeks the adults becomes sexually mature and female worms begin to lay eggs in the intestine that pass in the stool.[5]
Zoonotic Hookworm
Some species of zoonotic hookworm (i.e., cat and dog hookworms) include: Ancylostoma braziliense, Ancylostoma caninum, Ancylostoma ceylanicum and Uncinaria stenocephala. Among these most commonly encountered hookwormis Ancylostoma braziliense.[8] It causes cutaneous larva migrans (creeping eruption) generated by the larva migrating through the epidermis. This lesion is self-limiting, characterized by the erythematous serpiginous lesions.[9] Ancylostoma ceylanicum is the only species that develops to adult in humans, and causes enteric hookworm infection. Ancylostoma caninum occasionally reaches adulthood in humans, and causes eosinophilic enteritis.[10][11]
References
- ↑ Peduzzi R, Piffaretti JC (1983). "Ancylostoma duodenale and the Saint Gothard anaemia". Br Med J (Clin Res Ed). 287 (6409): 1942–5. doi:10.1136/bmj.287.6409.1942. PMC 1550193. PMID 6418279.
- ↑ Crompton DW, Whitehead RR (1993). "Hookworm infections and human iron metabolism". Parasitology. 107 Suppl: S137–45. doi:10.1017/s0031182000075569. PMID 8115178.
- ↑ Abuzeid AMI, Zhou X, Huang Y, Li G (2020). "Twenty-five-year research progress in hookworm excretory/secretory products". Parasit Vectors. 13 (1): 136. doi:10.1186/s13071-020-04010-8. PMC 7071665 Check
|pmc=value (help). PMID 32171305 Check|pmid=value (help). - ↑ Hawdon JM, Hotez PJ (1996). "Hookworm: developmental biology of the infectious process". Curr Opin Genet Dev. 6 (5): 618–23. doi:10.1016/s0959-437x(96)80092-x. PMID 8939719.
- ↑ 5.0 5.1 Jourdan PM, Lamberton PHL, Fenwick A, Addiss DG (2018). "Soil-transmitted helminth infections". Lancet. 391 (10117): 252–265. doi:10.1016/S0140-6736(17)31930-X. PMID 28882382.
- ↑ Loukas A, Hotez PJ, Diemert D, Yazdanbakhsh M, McCarthy JS, Correa-Oliveira R; et al. (2016). "Hookworm infection". Nat Rev Dis Primers. 2: 16088. doi:10.1038/nrdp.2016.88. PMID 27929101.
- ↑ Diemert DJ, Bethony JM, Hotez PJ (2008). "Hookworm vaccines". Clin Infect Dis. 46 (2): 282–8. doi:10.1086/524070. PMID 18171264.
- ↑ Bowman DD, Montgomery SP, Zajac AM, Eberhard ML, Kazacos KR (2010). "Hookworms of dogs and cats as agents of cutaneous larva migrans". Trends Parasitol. 26 (4): 162–7. doi:10.1016/j.pt.2010.01.005. PMID 20189454.
- ↑ Guill MA, Odom RB (1978). "Larva migrans complicated by Loeffler's syndrome". Arch Dermatol. 114 (10): 1525–6. PMID 718193.
- ↑ Tu CH, Liao WC, Chiang TH, Wang HP (2008). "Pet parasites infesting the human colon". Gastrointest Endosc. 67 (1): 159–60, commentary 160. doi:10.1016/j.gie.2007.07.009. PMID 17981278.
- ↑ Landmann JK, Prociv P (2003). "Experimental human infection with the dog hookworm, Ancylostoma caninum". Med J Aust. 178 (2): 69–71. doi:10.5694/j.1326-5377.2003.tb05222.x. PMID 12526725.