Acalabrutinib
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Yashasvi Aryaputra[2], Anmol Pitliya, M.B.B.S. M.D.[3]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Acalabrutinib is a kinase inhibitor that is FDA approved for the the treatment of mantle cell lymphoma (MCL). Common adverse reactions include anemia, thrombocytopenia, headache, neutropenia, diarrhea, fatigue, myalgia, and bruising.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications:
- Acalabrutinib is indicated for the treatment of adult patients with mantle cell lymphoma (MCL) who have received at least one prior therapy.
- This indication is approved under accelerated approval based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
Dose Modifications

Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding acalabrutinib Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding acalabrutinib Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Acalabrutinib FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding acalabrutinib Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding acalabrutinib Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
- None
Warnings
Hemorrhage
- Serious hemorrhagic events, including fatal events, have occurred in the combined safety database of 612 patients with hematologic malignancies treated with acalabrutinib monotherapy. Grade 3 or higher bleeding events, including gastrointestinal, intracranial, and epistaxis have been reported in 2% of patients. Overall, bleeding events including bruising and petechiae of any grade occurred in approximately 50% of patients with hematological malignancies.
- The mechanism for the bleeding events is not well understood. Acalabrutinib may further increase the risk of hemorrhage in patients receiving antiplatelet or anticoagulant therapies and patients should be monitored for signs of bleeding. Consider the benefit-risk of withholding acalabrutinib for 3-7 days pre- and post-surgery depending upon the type of surgery and the risk of bleeding.
Infection
- Serious infections (bacterial, viral or fungal), including fatal events and opportunistic infections have occurred in the combined safety database of 612 patients with hematologic malignancies treated with acalabrutinib monotherapy. Consider prophylaxis in patients who are at increased risk for opportunistic infections.
- Grade 3 or higher infections occurred in 18% of these patients. The most frequently reported Grade 3 or 4 infection was pneumonia. Infections due to hepatitis B virus (HBV) reactivation and progressive multifocal leukoencephalopathy (PML) have occurred. Monitor patients for signs and symptoms of infection and treat as medically appropriate.
Cytopenias
- In the combined safety database of 612 patients with hematologic malignancies, patients treated with acalabrutinib monotherapy experienced Grade 3 or 4 cytopenias, including neutropenia (23%), anemia (11%) and thrombocytopenia (8%) based on laboratory measurements. In the acalabrutinib clinical Trial LY-004, patients’ complete blood counts were assessed monthly during treatment.
Second Primary Malignancies
- Second primary malignancies, including non-skin carcinomas, have occurred in 11% of patients with hematologic malignancies treated with acalabrutinib monotherapy in the combined safety database of 612 patients. The most frequent second primary malignancy was skin cancer, reported in 7% of patients. Advise protection from sun exposure.
Atrial Fibrillation and Flutter
- In the combined safety database of 612 patients with hematologic malignancies treated with acalabrutinib monotherapy, atrial fibrillation and atrial flutter of any grade occurred in 3% of patients, and Grade 3 in 1% of patients. Monitor for atrial fibrillation and atrial flutter and manage as appropriate.
Adverse Reactions
Clinical Trials Experience
- As clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The safety data described in this section reflect exposure to acalabrutinib (100 mg twice daily) in 124 patients with previously treated MCL in Trial LY-004. The median duration of treatment with acalabrutinib was 16.6 (range 0.1 to 26.6) months. A total of 91 (73.4%) patients were treated with acalabrutinib for ≥ 6 months and 74 (59.7%) patients were treated for ≥ 1 year.
- The most common adverse reactions (≥ 20%) of any grade were anemia, thrombocytopenia, headache, neutropenia, diarrhea, fatigue, myalgia, and bruising. Grade 1 severity for the non-hematologic, most common events were as follows: headache (25%), diarrhea (16%), fatigue (20%), myalgia (15%), and bruising (19%). The most common Grade ≥ 3 non-hematological adverse reaction (reported in at least 2% of patients) was diarrhea.
- Dose reductions or discontinuation due to any adverse reaction were reported in 1.6% and 6.5% of patients, respectively.
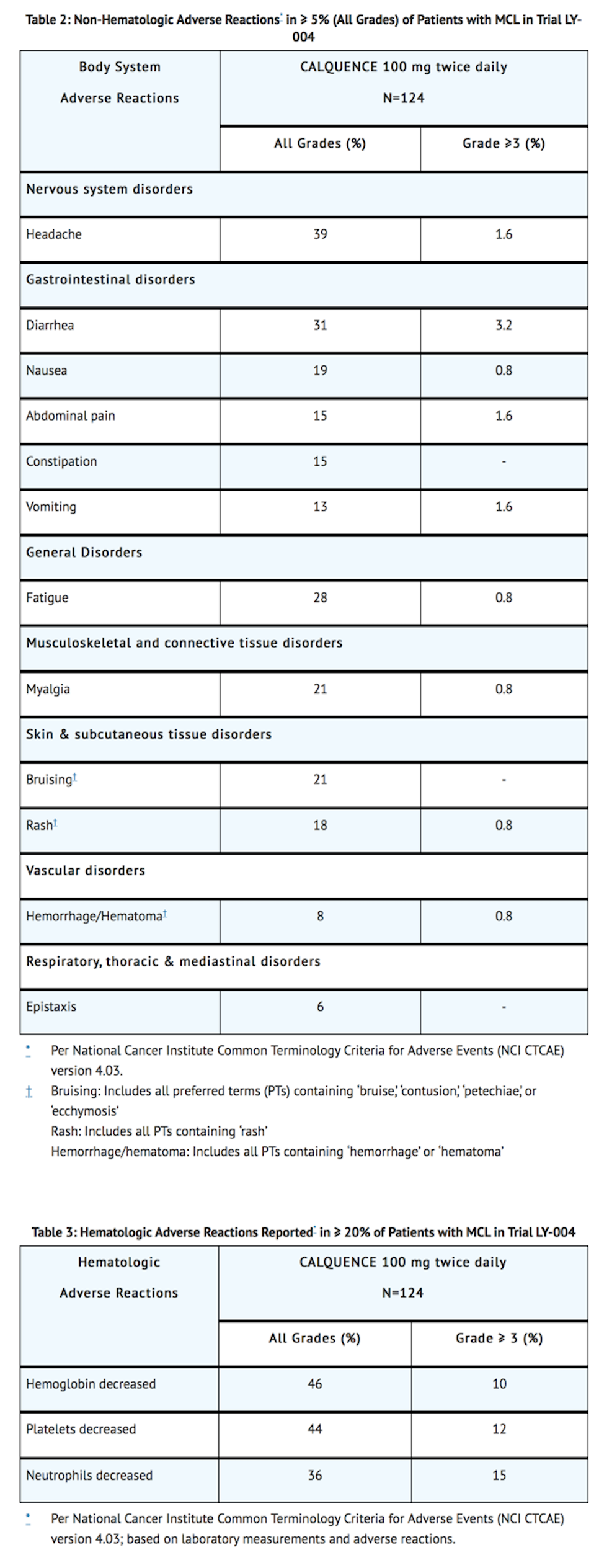
- Increases in creatinine 1.5 to 3 times the upper limit of normal occurred in 4.8% of patients.
Postmarketing Experience
There is limited information regarding Acalabrutinib Postmarketing Experience in the drug label.
Drug Interactions
Use in Specific Populations
Pregnancy
- Risk Summary
- Based on findings in animals, acalabrutinib may cause fetal harm when administered to a pregnant woman. There are no available data in pregnant women to inform the drug-associated risk. In animal reproduction studies, administration of acalabrutinib to pregnant rabbits during organogenesis resulted in reduced fetal growth at maternal exposures (AUC) approximately 4 times exposures in patients at the recommended dose of 100 mg twice daily (see Data). Advise pregnant women of the potential risk to a fetus.
- The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
- Data (Animal Data)
- In a combined fertility and embryo-fetal development study in female rats, acalabrutinib was administered orally at doses up to 200 mg/kg/day starting 14 days prior to mating through gestational day [GD] 17. No effects on embryo-fetal development and survival were observed. The AUC at 200 mg/kg/day in pregnant rats was approximately 16-times the AUC in patients at the recommended dose of 100 mg twice daily. The presence of acalabrutinib and its active metabolite were confirmed in fetal rat plasma.
- In an embryo-fetal development study in rabbits, pregnant animals were administered acalabrutinib orally at doses up to 200 mg/kg/day during the period of organogenesis (from GD 6-18). Administration of acalabrutinib at doses ≥ 100 mg/kg/day produced maternal toxicity and 100 mg/kg/day resulted in decreased fetal body weights and delayed skeletal ossification. The AUC at 100 mg/kg/day in pregnant rabbits was approximately 4-times the AUC in patients at 100 mg twice daily.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Acalabrutinib in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Acalabrutinib during labor and delivery.
Nursing Mothers
- No data are available regarding the presence of acalabrutinib or its active metabolite in human milk, its effects on the breastfed child, or on milk production. Acalabrutinib and its active metabolite were present in the milk of lactating rats. Due to the potential for adverse reactions in a breastfed child from acalabrutinib, advise lactating women not to breastfeed while taking acalabrutinib and for at least 2 weeks after the final dose.
Pediatric Use
- The safety and efficacy of acalabrutinib in pediatric patients have not been established.
Geriatic Use
- Eighty (64.5%) of the 124 MCL patients in clinical trials of acalabrutinib were 65 years of age or older, and 32 patients (25.8%) were 75 years of age or older. No clinically relevant differences in safety or efficacy were observed between patients ≥ 65 years and younger.
Gender
There is no FDA guidance on the use of Acalabrutinib with respect to specific gender populations.
Race
There is no FDA guidance on the use of Acalabrutinib with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Acalabrutinib in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Acalabrutinib in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Acalabrutinib in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Acalabrutinib in patients who are immunocompromised.
Administration and Monitoring
Administration
- The recommended dose of acalabrutinib is 100 mg taken orally approximately every twelve hours until disease progression or unacceptable toxicity.
- Advise patients to swallow capsule whole with water. Advise patients not to open, break or chew the capsules. Acalabrutinib may be taken with or without food. If a dose of acalabrutinib is missed by more than 3 hours, it should be skipped and the next dose should be taken at its regularly scheduled time. Extra capsules of acalabrutinib should not be taken to make up for a missed dose.
Monitoring
There is limited information regarding Acalabrutinib Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Acalabrutinib and IV administrations.
Overdosage
There is limited information regarding Acalabrutinib overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
| |
Acalabrutinib
| |
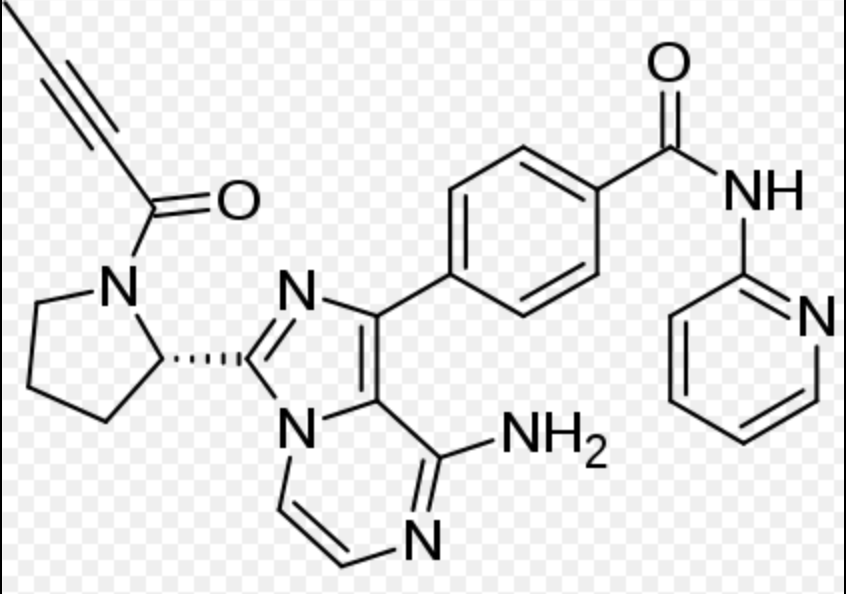
| Systematic (IUPAC) name | |
| 4-{8-Amino-3-[(2S)-1-(2-butynoyl)-2-pyrrolidinyl]imidazo[1,5-a]pyrazin-1-yl}-N-(2-pyridinyl)benzamide | |
| Identifiers | |
| CAS number | |
| ATC code | ? |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 465.507 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
- Acalabrutinib is a small-molecule inhibitor of BTK. Acalabrutinib and its active metabolite, ACP-5862, form a covalent bond with a cysteine residue in the BTK active site, leading to inhibition of BTK enzymatic activity. BTK is a signaling molecule of the B cell antigen receptor (BCR) and cytokine receptor pathways. In B cells, BTK signaling results in activation of pathways necessary for B-cell proliferation, trafficking, chemotaxis, and adhesion. In nonclinical studies, acalabrutinib inhibited BTK-mediated activation of downstream signaling proteins CD86 and CD69 and inhibited malignant B-cell proliferation and survival.
Structure

Pharmacodynamics
- In patients with B-cell malignancies dosed with 100 mg twice daily, median steady state BTK occupancy of ≥ 95% in peripheral blood was maintained over 12 hours, resulting in inactivation of BTK throughout the recommended dosing interval.
Cardiac Electrophysiology
- The effect of acalabrutinib on the QTc interval was evaluated in a randomized, double-blind, double-dummy, placebo- and positive-controlled, 4-way crossover thorough QTc study in 48 healthy adult subjects. Administration of a single dose of acalabrutinib that is the 4-fold maximum recommended single dose did not prolong the QTc interval to any clinically relevant extent (i.e., ≥ 10 ms).
Pharmacokinetics
- The pharmacokinetics (PK) of acalabrutinib was studied in healthy subjects and patients with B-cell malignancies. Acalabrutinib exhibits almost linear PK across a dose range of 75 to 250 mg (0.75 to 2.5 times the approved recommended single dose) and exhibits dose-proportionality. The daily area under the plasma drug concentration over time curve (AUC) was 1111 ng•h/mL and maximum plasma concentration (Cmax) of acalabrutinib was 323 ng/mL.
Absorption
- The geometric mean absolute bioavailability of acalabrutinib was 25%. Median time to peak acalabrutinib plasma concentrations (Tmax) was 0.75 hours.
Effect of Food
- In healthy subjects, administration of a single 75 mg dose of acalabrutinib (0.75 times the approved recommended single dose) with a high-fat, high-calorie meal (approximately 918 calories, 59 grams carbohydrate, 59 grams fat, and 39 grams protein) did not affect the mean AUC as compared to dosing under fasted conditions. Resulting Cmax decreased by 73% and Tmax was delayed 1-2 hours.
Distribution
- Reversible binding of acalabrutinib to human plasma protein was 97.5%. The in vitro mean blood-to-plasma ratio was 0.7. The mean steady-state volume of distribution (Vss) was approximately 34 L.
Elimination
- Following a single oral dose of 100 mg acalabrutinib, the median terminal elimination half-life (t1/2) of acalabrutinib was 0.9 (range: 0.6 to 2.8) hours. The t1/2 of the active metabolite, ACP-5862, was 6.9 hours.
- Acalabrutinib mean apparent oral clearance (CL/F) was 159 L/hr with similar PK between patients and healthy subjects, based on population PK analysis.
Metabolism
- Acalabrutinib is predominantly metabolized by CYP3A enzymes, and to a minor extent, by glutathione conjugation and amide hydrolysis, based on in vitro studies. ACP-5862 was identified as the major active metabolite in plasma with a geometric mean exposure (AUC) that was approximately 2- to 3-fold higher than the exposure of acalabrutinib. ACP-5862 is approximately 50% less potent than acalabrutinib with regard to BTK inhibition.
Excretion
- Following administration of a single 100 mg radiolabeled acalabrutinib dose in healthy subjects, 84% of the dose was recovered in the feces and 12% of the dose was recovered in the urine, with less than 1% of the dose excreted as unchanged acalabrutinib.
Specific Populations
Age, Race, and Body Weight
- Age (42 to 90 years), sex, race (Caucasian, African American), and body weight did not have clinically meaningful effects on the PK of acalabrutinib, based on population PK analysis.
Renal Impairment
- Acalabrutinib undergoes minimal renal elimination. Based on population PK analysis, no clinically relevant PK difference was observed in 368 patients with mild or moderate renal impairment (eGFR ≥ 30 mL/min/1.73m2, as estimated by MDRD (modification of diet in renal disease equation)). Acalabrutinib PK has not been evaluated in patients with severe renal impairment (eGFR < 29 mL/min/1.73m2, MDRD) or renal impairment requiring dialysis.
Hepatic Impairment
- Acalabrutinib is metabolized in the liver. In a hepatic impairment study, compared to subjects with normal liver function (n=6), acalabrutinib exposure (AUC) was increased by less than two-fold in subjects with mild (n=6) (Child-Pugh A) and moderate (n=6) (Child-Pugh B) hepatic impairment, respectively. Based on a population PK analysis, no clinically relevant PK difference was observed in subjects with mild (n=41) or moderate (n=3) hepatic impairment (total bilirubin between 1.5 to 3 times the upper limit of normal [ULN] and any AST) relative to subjects with normal (n=527) hepatic function (total bilirubin and AST within ULN). Acalabrutinib PK has not been evaluated in patients with severe hepatic impairment (Child-Pugh C or total bilirubin between 3 and 10 times ULN and any AST).
Drug Interaction Studies
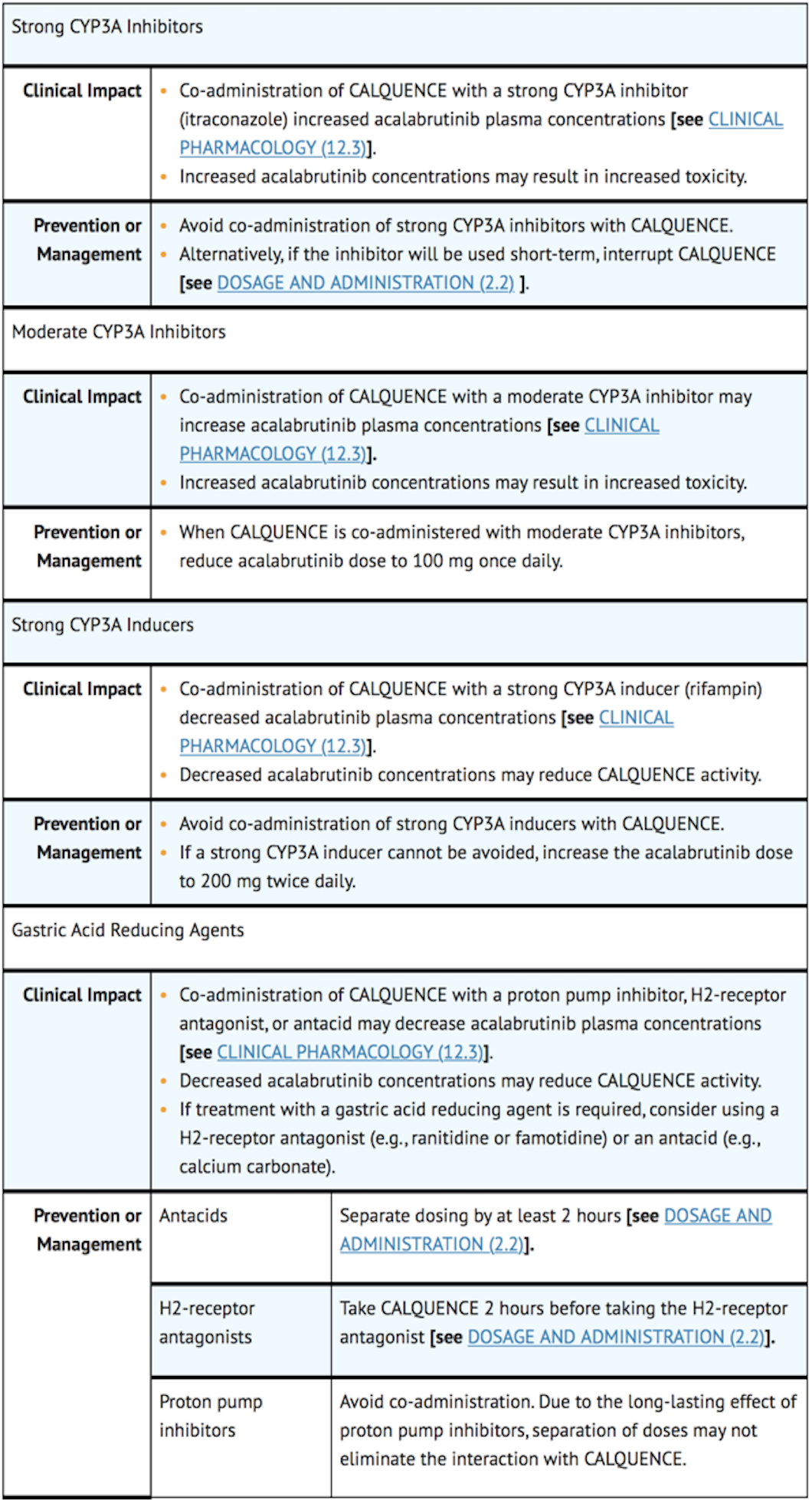
Effect of CYP3A Inhibitors on acalabrutinib
- Co-administration with a strong CYP3A inhibitor (200 mg itraconazole once daily for 5 days) increased the acalabrutinib Cmax by 3.9-fold and AUC by 5.1-fold in healthy subjects.
- Physiologically based pharmacokinetic (PBPK) simulations with acalabrutinib and moderate CYP3A inhibitors (erythromycin, fluconazole, diltiazem) showed that co-administration increased acalabrutinib Cmax and AUC increased by 2- to almost 3-fold.
Effect of CYP3A Inducers on acalabrutinib
- Co-administration with a strong CYP3A inducer (600 mg rifampin once daily for 9 days) decreased acalabrutinib Cmax by 68% and AUC by 77% in healthy subjects.
Gastric Acid Reducing Agents
- Acalabrutinib solubility decreases with increasing pH. Co-administration with an antacid (1 g calcium carbonate) decreased acalabrutinib AUC by 53% in healthy subjects. Co-administration with a proton pump inhibitor (40 mg omeprazole for 5 days) decreased acalabrutinib AUC by 43%.
In Vitro Studies
Metabolic Pathways
- Acalabrutinib is a weak inhibitor of CYP3A4/5, CYP2C8 and CYP2C9, but does not inhibit CYP1A2, CYP2B6, CYP2C19, and CYP2D6. The active metabolite (ACP-5862) is a weak inhibitor of CYP2C8, CYP2C9 and CYP2C19, but does not inhibit CYP1A2, CYP2B6, CYP2D6 and CYP3A4/5.
- Acalabrutinib is a weak inducer of CYP1A2, CYP2B6 and CYP3A4; the active metabolite (ACP-5862) weakly induces CYP3A4.
- Based on in vitro data and PBPK modeling, no interaction with CYP substrates is expected at clinically relevant concentrations.
Drug Transporter Systems
- Acalabrutinib is a substrate of P-glycoprotein (P-gp) and BCRP. Acalabrutinib is not a substrate of renal uptake transporters OAT1, OAT3, and OCT2, or hepatic transporters OATP1B1, and OATP1B3.
- Acalabrutinib does not inhibit P-gp, OAT1, OAT3, OCT2, OATP1B1, and OATP1B3 at clinically relevant concentrations.
- Acalabrutinib may increase exposure to co-administered BCRP substrates (e.g., methotrexate) by inhibition of intestinal BCRP.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenicity studies have not been conducted with acalabrutinib.
- Acalabrutinib was not mutagenic in an in vitro bacterial reverse mutation (AMES) assay or clastogenic in an in vitro human lymphocyte chromosomal aberration assay or in an in vivo rat bone marrow micronucleus assay.
- In a fertility study in rats, there were no effects of acalabrutinib on fertility in male rats at exposures 18-times, or in female rats at exposures 16-times the AUC observed in patients at the recommended dose of 100 mg twice daily.
Clinical Studies
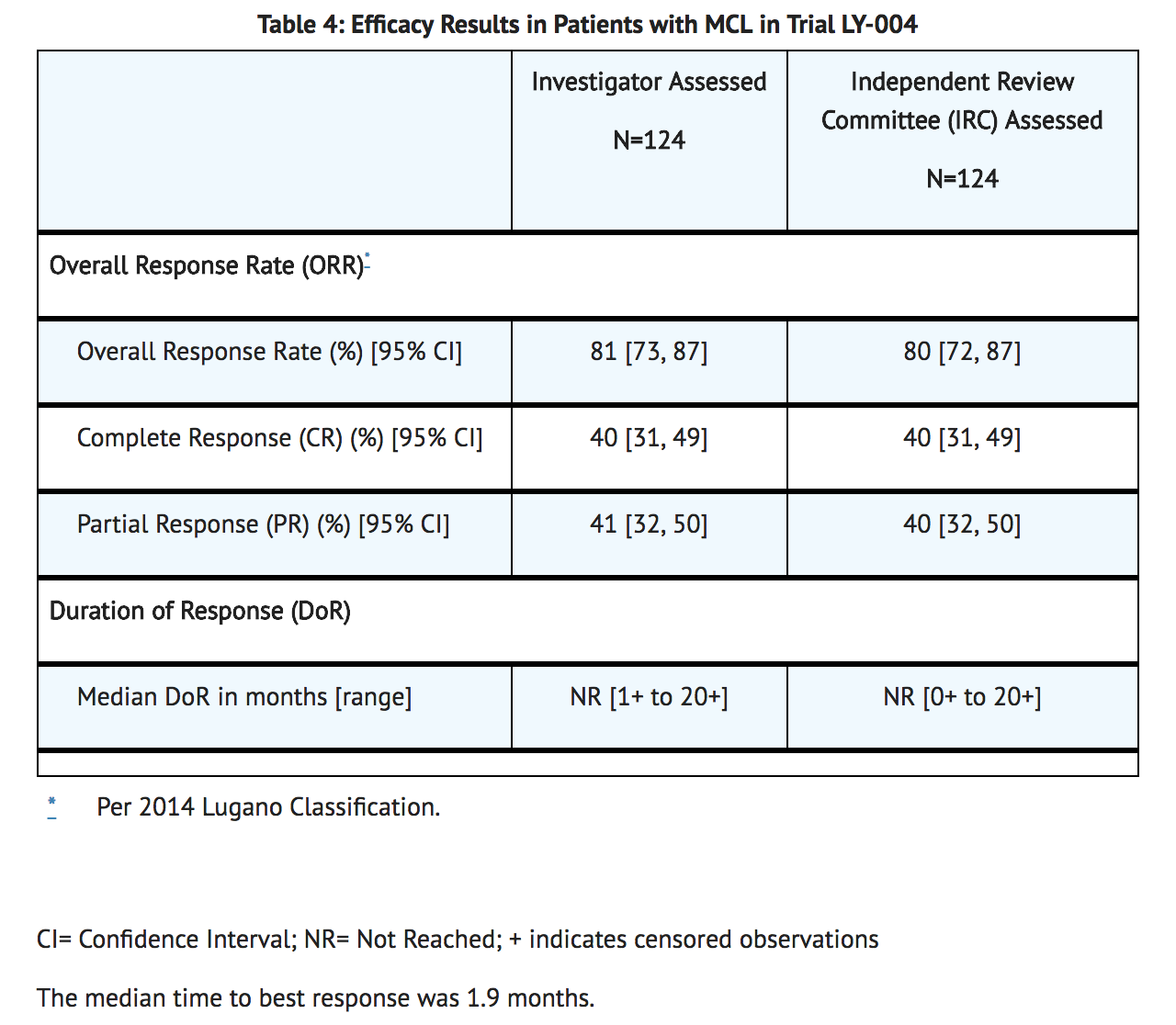
- The efficacy of acalabrutinib was based upon Trial LY-004 titled “An Open-label, Phase 2 Study of ACP-196 in Subjects with Mantle Cell Lymphoma” (NCT02213926). Trial LY-004 enrolled a total of 124 patients with MCL who had received at least one prior therapy.
- The median age was 68 (range 42 to 90) years, 80% were male, and 74% were Caucasian. At baseline, 93% of patients had an ECOG performance status of 0 or 1. The median time since diagnosis was 46.3 months and the median number of prior treatments was 2 (range 1 to 5), including 18% with prior stem cell transplant. Patients who received prior treatment with BTK inhibitors were excluded. The most common prior regimens were CHOP-based (52%) and ARA-C (34%). At baseline, 37% of patients had at least one tumor with a longest diameter ≥ 5 cm, 73% had extra nodal involvement including 51% with bone marrow involvement. The simplified MIPI score (which includes age, ECOG score, and baseline lactate dehydrogenase and white cell count) was intermediate in 44% and high in 17% of patients.
- Acalabrutinib was administered orally at 100 mg twice daily until disease progression or unacceptable toxicity. The median dose intensity was 98.5%. Tumor response was assessed according to the Lugano Classification for Non-Hodgkin’s lymphoma (NHL). The major efficacy outcome of Trial LY-004 was overall response rate (ORR) and the median follow-up was 15.2 months.
Lymphocytosis
- Upon initiation of acalabrutinib, a temporary increase in lymphocyte counts (defined as absolute lymphocyte count (ALC) increased ≥ 50% from baseline and a post baseline assessment ≥ 5 x 109) in 31.5% of patients in Trial LY-004. The median time to onset of lymphocytosis was 1.1 weeks and the median duration of lymphocytosis was 6.7 weeks.
How Supplied
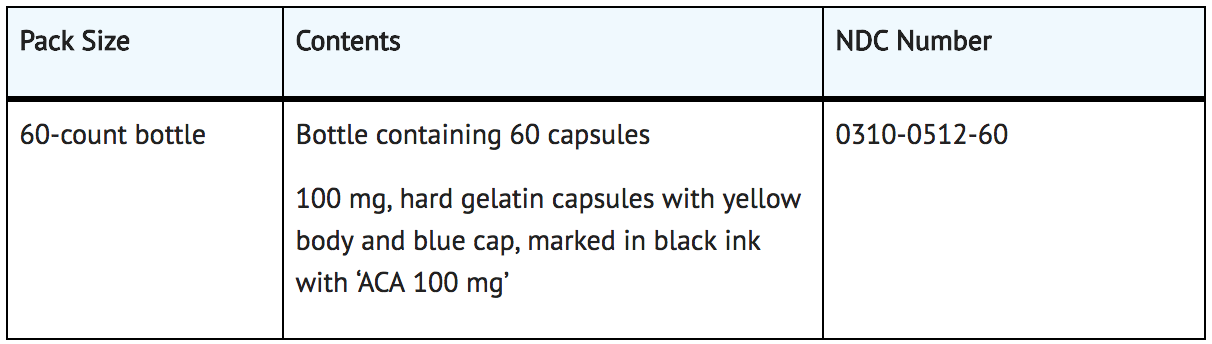
Storage
- Store at 20°C-25°C (68°F-77°F); excursions permitted to 15°C-30°C (59°F- 86°F).
Images
Drug Images
{{#ask: Page Name::Acalabrutinib |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Acalabrutinib |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Hemorrhage
- Inform patients to report signs or symptoms of severe bleeding. Inform patients that acalabrutinib may need to be interrupted for major surgeries.
Infections
- Inform patients to report signs or symptoms suggestive of infection.
Cytopenias
- Inform patients that they will need periodic blood tests to check blood counts during treatment with acalabrutinib.
Second Primary Malignancies
- Inform patients that other malignancies have been reported in patients who have been treated with acalabrutinib, including skin cancer. Advise patients to use sun protection.
Atrial Fibrillation and Flutter
- Counsel patients to report any signs of palpitations, lightheadedness, dizziness, fainting, shortness of breath, and chest discomfort.
Dosing Instructions
- Instruct patients to take acalabrutinib orally twice daily, about 12 hours apart. Acalabrutinib may be taken with or without food. Advise patients that acalabrutinib capsules should be swallowed whole with a glass of water, without being opened, broken, or chewed.
Missed Dose
- Advise patients that if they miss a dose of acalabrutinib, they may still take it up to 3 hours after the time they would normally take it. If more than 3 hours have elapsed, they should be instructed to skip that dose and take their next dose of acalabrutinib at the usual time. Warn patients they should not take extra capsules to make up for the dose that they missed.
Drug Interactions
- Advise patients to inform their healthcare providers of all concomitant medications, including over-the-counter medications, vitamins and herbal products.
Lactation
- Advise women not to breastfeed during treatment with acalabrutinib and for at least 2 weeks after the final dose.
Precautions with Alcohol
Alcohol-Acalabrutinib interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Calquence
Look-Alike Drug Names
There is limited information regarding Acalabrutinib Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.