Abciximab (patient information)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Generic Name: Abciximab
Trade Name: ReoPro
For intravenous administration
DESCRIPTION:
Abciximab, ReoPro @ , is the Fab fragment of the chimeric human-murine monoclonal antibody 7E3. Abciximab binds to the glycoprotein (GP) IIb/IIIa receptor of human platelets and inhibits platelet aggregation. The chimeric 7E3 antibody is produced by continuous perfusion in mammalian cell culture. The 47,615 dalton Fab fragment is purified from cell culture supematant by a series of steps involving specific viral inactivation and removal procedures, digestion with papain and column chromatography. ReoPro@ is a clear, colorless, sterile, non-pyrogenic solution for intravenous (IV) use. Each single use vial contains 2 mg/mL of Abciximab in a buffered solution (pH 7.2) of 0.01 M sodium phosphate, 0.15 M sodium chloride and 0.00 1% polysorbate 80 in Water for Injection. No preservatives are added.
CLINICAL PHARMACOLOGY:
General
Abciximab binds to the intact platelet GPIIb/IIIa receptor, which is a member of the integrin family of adhesion receptors and the major platelet surface receptor involved in platelet aggregation. Abciximab inhibits platelet aggregation by preventing the binding of fibrinogen, von Willebrand factor, and other adhesive molecules to GPIIb/IIIa receptor sites on activated platelets. The mechanism of action is thought to involve steric hindrance and/or conformational effects to block access of large molecules to the receptor rather than direct interaction with the RGD (arginine-glycine-aspartic acid) binding site of GPIIb/IIIa.
Pre-clinical experience
Maximal inhibition of platelet aggregation was observed when r: 80% of GPIIb/IIIa receptors were blocked .by Abciximab. In non-human primates, Abciximab bolus doses of 0.25 mg/kg generally achieved a blockade of at least 80% of platelet receptors and fully inhibited platelet aggregation. Inhibition of platelet function was temporary following a bolus dose, but receptor blockade could be sustained at ~80% by continuous intravenous infusion. The inhibitory effects of Abciximab were substantially reversed by the transfusion of platelets in monkeys. The antithrombotic efficacy of prototype antibodies [murine 7E3 Fab and F(ab’)z] and Abciximab was evaluated in dog, monkey and baboon models of coronary, carotid, and femoral artery thrombosis. Doses of the murine version of 7E3 or Abciximab sufficient to produce high-grade (2 80%) GPIIb/IIIa receptor blockade prevented acute thrombosis and yielded lower rates of thrombosis compared with aspirin and/or heparin.
Pharmacokinetics
Following intravenous bolus administration, free plasma concentrations of Abciximab decrease rapidly with an initial half-life of less than 10 minutes and a second phase half-life of about 30 minutes, probably related to rapid binding to the platelet GPIIb/IIIa receptors. Platelet function generally recovers over the course of 48 hours (1,2), although Abciximab remains in the circulation for 15 days or more in a platelet-bound state. Intravenous administration of a 0.25 mg/kg bolus dose of Abciximab followed by continuous infusion of 10 ,ug/min (or a weight-adjusted infusion of 0.125 l.tg/kg/min to a maximum of 10 pg/min) produces approximately constant free plasma concentrations throughout the infusion. At the termination of the infusion period, free plasma concentrations fall rapidly for approximately six hours then decline at a slower rate.
Pharmacodynamics
Intravenous administration in humans of single bolus doses of Abciximab from 0.15 mg/kg to 0.30 mg/kg produced rapid dose-dependent inhibition of platelet function as measured by ex vivo platelet aggregation in response to adenosine diphosphate (ADP) or by prolongation of bleeding time. At the two highest doses (0.25 and 0.30 mg/kg) at two hours post injection, over 80% of the GPIIb/IIIa receptors were blocked and platelet aggregation in response to 20 pM ADP was almost abolished. The median bleeding time increased to over 30 minutes at both doses compared with a baseline value of approximately five minutes. Intravenous administration in humans of a single bolus dose of 0.25 mg/kg followed by a continuous infusion of 10 pg/min for periods of 12 to 96 hours produced sustained high-grade GPIIb/IIIa receptor blockade (2 80%) and inhibition of platelet function (ex vivo platelet aggregation in response to 5 pM or 20 pM ADP less than 20% of baseline and bleeding time greater than 30 minutes) for the duration of the infusion in most patients. Similar results were obtained when a weight-adjusted infusion dose (0.125 &kg/min to a maximum of 10 pg/min) was used in patients weighing up to 80 kg. Results in patients who received the 0.25 mg/kg bolus followed by a 5 pg/min infusion for 24 hours showed a similar initial receptor blockade and inhibition of platelet aggregation, but the response was not maintained throughout the infusion period. Low levels of GPIIb/IIIa receptor blockade are present for more than 10 days following cessation of the infusion. After discontinuation of Abciximab infusion, platelet function returns gradually to normal. Bleeding time returned to _< 12 minutes within 12 hours following the end of infusion in 15 of 20 patients (75%), and within 24 hours in 18 of 20 patients (90%). Ex viva platelet a ggregation in response to 5 pM ADP returned to 2 50% of baseline within 24 hours following the end of infusion in 11 of 32 patients (34%) and within 48 hours in 23 of 32 patients (72%). In response to 20 pM ADP, ex vivo platelet aggregation returned to 2 50% of baseline within 24 hours in 20 of 32 patients (62%) and within 48 hours in 28 of 32 patients (88%).
CLINICAL STUDIES:
Abciximab has been studied in three Phase 3 clinical trials, all of which evaluated the effect of Abciximab in patients undergoing percutaneous coronary intervention: in patients at high risk for abrupt closure of the treated coronary vessel (EPIC), in a broader group of patients (EPILOG), and in unstable angina patients not responding to conventional medical therapy (CAPTURE). Percutaneous intervention included balloon angioplasty, atherectomy, or stent placement. All trials involved the use of various, concomitant heparin dose regimens and, unless contraindicated, aspirin (325 mg) was administered orally two hours prior to the planned procedure and then once daily. EPIC was a multicenter, double-blind, placebo-controlled trial of Abciximab in patients undergoing percutaneous transluminal coronary angioplasty or atherectomy (3). In the EPIC trial, 2099 patients between 26 and 83 years of age who were at high risk for abrupt closure of the treated coronary vessel were randomly allocated to one of three treatments: 1) an Abciximab bolus (0.25 mg/kg) followed by an Abciximab infusion (10 pg/min) for 12 hours (bolus plus infusion group); 2) an Abciximab bolus (0.25 mg/kg) followed by a placebo infusion (bolus group), or; 3) a placebo bolus followed by a placebo infusion (placebo group). Patients at high risk during or following percutaneous coronary intervention were defined as those with unstable angina or non-Q wave myocardial infarction (n=489), those with an acute Q-wave myocardial infarction within 12 hours of symptom onset (n=66), and those who were at high risk because of coronary morphology and/or clinical characteristics (n=1.544). Trea tment with study agent in each of the three arms was initiated IO-60 minutes before the onset of percutaneous coronary intervention. All patients initially received an intravenous heparin bolus (10,000 to 12,000 units) and boluses of up to 3,000 units thereafter to a maximum of 20,000 units during percutaneous coronary intervention. Heparin infusion was continued for 12 hours to maintain a therapeutic elevation of activated partial thromboplastin time (APTT, 1.5-2.5 times normal).
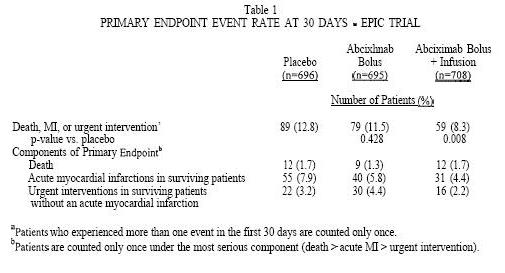
The primary endpoint was the occurrence of any of the following events within 30 days of percutaneous coronary intervention: death, myocardial infarction (MI), or the need for urgent intervention for recurrent ischemia [i.e., urgent percutaneous transluminal coronary angioplasty, urgent coronary artery bypass graft (CABG) surgery, a coronary stent, or an intra-aortic balloon pump]. The 30-day (Kaplan-Meier) primary endpoint event rates for each treatment group by intention-to-treat analysis of all randomized patients are shown in Table 1. The 4.5% lower incidence of the primary endpoint rates in the bolus plus infusion treatment group, compared with the placebo group, was statistically significant, whereas the 1.3% lower incidence in the bolus treatment group was not. A lower incidence of the primary endpoint was observed in the bolus plus infusion treatment arm for all three high-risk subgroups: patients with unstable angina, patients presenting within 12 hours of the onset of symptoms of an acute myocardial infarction, and patients with other high-risk clinical and/or morphologic characteristics (3). The treatment effect was largest in the first two subgroups and smallest in the third subgroup.
The primary endpoint event rates in the bolus plus infusion treatment group were reduced mostly in the first 48 hours and this benefit was sustained through blinded evaluations at 30 days(3), and six months(4). At the sixmonth follow-up visit this event rate remained lower in the bolus plus infusion arm (12.3%) than in the placebo arm (17.6%) (p=O.O06 vs. placebo).
EPILOG was a randomized, double-blind, multicenter, placebo-controlled trial which evaluated Abciximab in a broad population of patients undergoing percutaneous coronary intervention (excluding patients with myocardial infarction and unstable angina meeting the EPIC high risk criteria)(5). EPILOG tested the hypothesis that use of a low-dose, weight-adjusted heparin regimen, early femoral arterial sheath removal, improved access site management and weight-adjustment of the Abciximab infusion dose could significantly lower the bleeding rate yet maintain the efficacy seen in the EPIC trial. EPILOG was a three treatment-arm trial: Abciximab plus standard dose, weight-adjusted heparin’; Abciximab plus low dose, weight-adjusted heparin2; and placebo plus standard dose, weight-adjusted heparin. The Abciximab bolus dose was the same as that used in the EPIC trial ’ Bolus administration of 100 U/kg weight-adjusted heparin to achieve an activated clotting time (ACT) of 300 seconds (maximum initial bolus 10,000 units). ’ Bolus administration of 70 U/kg weight-adjusted heparin to achieve an activated clotting time (ACT) of 200 seconds (maximum initial bolus 7,000 units).
(0.25 mg/kg), but the continuous infusion dose was weight adjusted in patients up to 80 kg3 (0.125 pg/kg/min). Specific patient and access site management procedures as well as a strong recommendation for early sheath removal were also incorporated into the trial as described in PRECAUTIONS. The EPILOG trial achieved the objective of lowering the bleeding rate while maintaining efficacy: in the Abciximab treatment arms major bleeding was not significantly different from that in the placebo arm (see ADVERSE REACTIONS: Bleeding).
The primary endpoint of the EPILOG trial was the composite of death or MI occurring within 30 days of percutaneous coronary intervention. The composite of death, MI, or urgent intervention was an important secondary endpoint. As seen in the EPIC trial, the endpoint events in the Abciximab treatment group were reduced mostly in the first 48 hours and this benefit was sustained through blinded evaluations at 30 days and six months. The (Kaplan-Meier) endpoint event rates at 30 days are shown in Table 2 for each treatment group by intention-to-treat analysis of all 2792 randomized patients. At the six-month follow-up visit, the event rate for death, MI, or repeat (urgent or non-urgent) intervention remained lower in the Abciximab treatment arms (22.3% and 22.8%, respectively, for the standard- and low-dose heparin arms) than in the placebo arm (25.8%) and the event rate for death, MI, or urgent intervention was substantially lower in the Abciximab treatment arms (8.3% and 8.4%, respectively, for the standard- and low-dose heparin arms) than in the placebo arm (14.7%). The proportionate reductions in endpoint event rates were similar irrespective of the type of coronary intervention used (balloon angioplasty, atherectomy, or stent placement). Risk assessment using the American College of Cardiology/American Heart Association clinical / morphological criteria had large inter-observer variability. Consequently, a low risk subgroup could not be reproducibly identified in which to evaluate efficacy.
CAPTURE was a randomized, double-blind, multicenter, placebo-controlled trial of the use of Abciximab in unstable angina patients not responding to conventional medical therapy for whom percutaneous coronary intervention was planned, but not immediately performed (6). In contrast to the EPIC and EPILOG trials, the CAPTURE trial involved the administration of placebo or Abciximab starting 18 to 24 hours prior to percutaneous coronary intervention and continuing until one hour after completion of the intervention.
Patients were assessed as having unstable angina not responding to conventional medical therapy if they had at least one episode of myocardial ischemia despite bed rest and at least two hours of therapy with intravenous heparin and oral or intravenous nitrates. These patients were enrolled into the CAPTURE trial, if during a screening angiogram, they were determined to have a coronary lesion amenable to percutaneous coronary intervention. Patients received a bolus dose and intravenous infusion of placebo or Abciximab for 18 to 24 hours. At the end of the infusion period, the intervention was performed. The Abciximab or placebo infusion was discontinued one hour following the intervention. Patients were treated with intravenous heparin and oral or intravenous nitrates throughout the 18 to 24-hour Abciximab infusion period prior to the percutaneous coronary intervention.
The Abciximab dose was a 0.25 mgkg bolus followed by a continuous infusion at a rate of 10 pg/min. The CAPTURE trial incorporated weight adjustment of the standard heparin dose only during the performance of the intervention, but did not investigate the effect of a lower heparin dose, and arterial sheaths were left in place for approximately 40 hours. The primary endpoint of the CAPTURE trial was the occurrence of any of the following events within 30 days of percutaneous coronary intervention: death, MI, or urgent intervention. The 30-day (Kaplan-Meier) primary endpoint event rates for each treatment group by intention-to-treat analysis of all 126.5 randomized patients are shown in Table 3.
The 30-day results are consistent with EPIC results, with the greatest effects on the myocardial infarction and urgent intervention components of the composite endpoint. As secondary endpoints, the components of the composite endpoint were analyzed separately for the period prior to the percutaneous coronary intervention and the period from the beginning of the intervention through Day 30. The greatest difference in MI occurred in the post-intervention period: the rates of MI were lower in the Abciximab group compared with placebo (Abciximab 3.6%, placebo 6.1%). There was also a reduction in MI occurring prior to the percutaneous coronary intervention (Abciximab 0.6%, placebo 2.0%). An Abciximab-associated reduction in the incidence of urgent intervention occurred in the post-intervention period. No effect on mortality was observed in either period. At six months of follow up, the composite endpoint of death, MI, or repeat intervention (urgent or non-urgent) was not different between the Abciximab and placebo groups (Abciximab 3 1 .O%, placebo 30.8%, p-0.77).
Mortality was uncommon in all three trials, EPIC, EPILOG and CAPTURE. Similar mortality rates were observed in all arms within each trial. In all three trials, the rates of acute MI were significantly lower in the groups treated with Abciximab. Urgent intervention rates were also lower in Abciximab-treated groups in these trials.
Anticoagulation: Due to the incidence of bleeding seen in the EPIC trial, the dosing regimens of concomitant heparin and the target levels for anticoagulation were successively varied in the CAPTURE and EPILOG trials. These modified dosing regimens combined with other measures for patient management were associated with reduced bleeding rates (see ADVERSE REACTIONS: Bleeding)
EPILOG trial: Heparin was weight adjusted in all treatment arms. A baseline ACT was determined prior to percutaneous coronary intervention. In the low-dose heparin arm of the trial, heparin was administered as follows: _ The initial heparin bolus was based upon the results of the baseline ACT, according to the following regimen: ACT < 150 seconds: administer 70 U/kg heparin ACT 150 - 199 seconds: administer 50 U/kg heparin ACT 2200 seconds: administer no heparin Additional 20 U/kg heparin boluses were given to achieve and maintain an ACT of 200 seconds during the procedure. Discontinuation of heparin immediately after the procedure and removal of the arterial sheath within six hours were strongly recommended in the trial. If prolonged heparin therapy or delayed sheath removal was clinically indicated, heparin was adjusted to keep the APTT at a target of 60 to 85 seconds.
CAPTURE trial: Anticoagulation was initiated prior to the administration of Abciximab. Anticoagulation was initiated with an intravenous heparin infusion to achieve a target APTT of 60 to 85 seconds. The heparin infusion was not uniformly weight adjusted in this trial. The heparin infusion was maintained during the Abciximab infusion and was adjusted to achieve an ACT of 300 seconds or an APTT of 70 seconds during the percutaneous coronary intervention. Following the intervention, heparin management was as outlined above for the EPILOG trial.
INDICATIONS AND USAGE:
Abciximab is indicated as an adjunct to percutaneous coronary intervention for the prevention of cardiac ischemic complications
- in patients undergoing percutaneous coronary intervention
- in patients with unstable angina not responding to conventional medical therapy when percutaneous coronary intervention is planned within 24 hours
Abciximab use in patients not undergoing percutaneous coronary intervention has not been studied.
Abciximab is intended for use with aspirin and heparin and has been studied only in that setting, as described in CLINICAL STUDIES.
CONTRAINDICATIONS:
Because Abciximab may increase the risk of bleeding, Abciximab is contraindicated in the following clinical situations:
- Active internal bleeding
- Recent (within six weeks) gastrointestinal (GI) or genitourinary (GU) bleeding of clinical significance.
- History of cerebrovascular accident (CVA) within two years, or CVA with a significant residual neurological deficit
- Bleeding diathesis
- Administration of oral anticoagulants within seven days unless prothrombin time is I 1.2 times control
- Thrombocytopenia (< 100,000 cells/pL)
- Recent (within six weeks) major surgery or trauma
- Intracranial neoplasm, arteriovenous malformation, or aneurysm
- Severe uncontrolled hypertension
- Presumed or documented history of vasculitis
- Use of intravenous dextran before percutaneous coronary intervention, or intent to use it during an intervention
Abciximab is also contraindicated in patients with known hypersensitivity to any component of this product or to murine proteins.
WARNINGS:
Abciximab has the potential to increase the risk of bleeding, particularly in the presence of anticoagulation, e.g.,from heparin, other anticoagulants, or thrombolytics (see ADVERSE REACTIONS: Bleeding).
The risk of major bleeds due to Abciximab therapy may be increased in patients receiving thrombolytics andshould be weighed against the anticipated benefits.
Should serious bleeding occur that is not controllable with pressure, the infusion of Abciximab and anyconcomitant heparin should be stopped.
PRECAUTIONS:
Bleeding Precautions
Results of the EPILOG trial show that bleeding can be reduced by the use of low-dose, weight-adjusted heparin regimens, adherence to stricter anticoagulation guidelines, early femoral arterial sheath removal, careful patient and access site management and weight-adjustment of the Abciximab infusion dose.
Therapy with Abciximab requires careful attention to all potential bleeding sites (including catheter insertion sites, arterial and venous puncture sites, cutdown sites, needle puncture sites, and gastrointestinal, genitourinary, and retroperitoneal sites).
Arterial and venous punctures, intramuscular injections, and use of urinary catheters, nasotracheal intubation, nasogastric tubes and automatic blood pressure cuffs should be minimized. When obtaining intravenous access, non-compressible sites (e.g., subclavian or jugular veins) should be avoided. Saline or heparin locks should be considered for blood drawing. Vascular puncture sites should be documented and monitored. Gentle care should be provided when removing dressings.
Femoral artery access site:
Arterial access site care is important to prevent bleeding. Care should be taken when attempting vascular access that only the anterior wall of the femoral artery is punctured, avoiding a Seldinger (through and through) technique for obtaining sheath access. Femoral vein sheath placement should be avoided unless needed. While the vascular sheath is in place, patients should be maintained on complete bed rest with the head of the bed 130” and the affected limb restrained in a straight position. Patients may be medicated for bacWgroin pain as necessary.
Discontinuation of heparin immediately upon completion of the procedure and removal of the arterial sheath within six hours is strongly recommended if APTT I 50 set or ACT < 175 set (See PRECAUTIONS: Laboratory Tests). In all circumstances, heparin should be discontinued at least two hours prior to arterial sheath removal.
Following sheath removal, pressure should be applied to the femoral artery for at least 30 minutes using either manual compression or a mechanical device for hemostasis. A pressure dressing should be applied following hemostasis. The patient should be maintained on bed rest for six to eight hours following sheath removal or discontinuation of Abciximab, or four hours following discontinuation of heparin, whichever is later. The pressure dressing should be removed prior to ambulation. The sheath insertion site and distal pulses of affected leg(s) should be frequently checked while the femoral artery sheath is in place and for six hours after femoral artery sheath removal. Any hematoma should be measured and monitored for enlargement.
The following conditions have been associated with an increased risk of bleeding and may be additive with the effect of Abciximab in the angioplasty setting: percutaneous coronary intervention within 12 hours of the onset of symptoms for acute myocardial infarction, prolonged percutaneous coronary intervention (lasting more than 70 minutes) and failed percutaneous coronary intervention.
Use of Thrombolytics, Anticoagulants and Other Antiplatelet Agents
In the EPIC, EPILOG and CAPTURE trials, Abciximab was used concomitantly with heparin and aspirin. For details of the anticoagulation algorithms used in these clinical trials, see CLINICAL STUDIES: Anticoagulation. Because Abciximab inhibits platelet aggregation, caution should be employed when it is used with other drugs that affect hemostasis, including thrombolytics, oral anticoagulants, non-steroidal anti-inflammatory drugs, dipyridamole, and ticlopidine.
In the EPIC trial, there was limited experience with the administration of Abciximab with low molecular weight dextran. Low molecular weight dextran was usually given for the deployment of a coronary stent, for which oral anticoagulants were also given. In the 11 patients who received low molecular weight dextran with Abciximab, five had major bleeding events and four had minor bleeding events. None of the five placebo patients treated with low molecular weight dextran had a major or minor bleeding event (see CONTRAINDICATIONS).
There are limited data on the use of Abciximab in patients receiving thrombolytic agents. Because of concern about synergistic effects on bleeding, systemic thrombolytic therapy should be used judiciously.
Thrombocytopenia
Platelet counts should be monitored prior to treatment, two to four hours following the bolus dose of Abciximab and at 24 hours or prior to discharge, whichever is first. If a patient experiences an acute platelet decrease (e.g., a platelet decrease to less than 100,000 cells/pL and a decrease of at least 25% from pre-treatment value), additional platelet counts should be determined. These platelet counts should be drawn in three separate tubes containing ethylenediaminetetraacetic acid (EDTA), citrate and heparin, respectively, to exclude pseudothrombocytopenia due to in vitro anticoagulant interaction. If true thrombocytopenia is verified, Abciximab should be immediately discontinued and the condition appropriately monitored and treated. For patients with thrombocytopenia in the clinical trials, a daily platelet count was obtained until it returned to normal. If a patient’s platelet count dropped to 60,000 cells/pL, heparin and aspirin were discontinued. If a patient’s platelet count dropped below 50,000 cells/FL, platelets were transfused. Most cases of severe thrombocytopenia (<50,000 cells/pL) occurred within the first 24 hours of Abciximab administration.
Restoration of Platelet Function
In the event of serious uncontrolled bleeding or the need for emergency surgery, Abciximab should be discontinued. If platelet function does not return to normal, it may be restored, at least in part, with platelet transfusions.
Laboratory Tests
Before infusion of Abciximab, platelet count, prothrombin time, ACT and APTT should be measured to identify pre-existing hemostatic abnormalities. Based on an integrated analysis of data from all studies, the following guidelines may be utilized to minimize the risk for bleeding: When Abciximab is initiated 18 to 24 hours before percutaneous coronary intervention, the APTT should_ be maintained between 60 and 85 seconds during the Abciximab and heparin infusion period. During percutaneous coronary intervention the ACT should be maintained between 200 and 300 seconds. If anticoagulation is continued in these patients following percutaneous coronary intervention, the APTT should be maintained between 60 and 85 seconds. The AP?T or ACT should be checked prior to arterial sheath removal. The sheath should not be removed unless APTT I 50 seconds or ACT I 175 seconds.
Readministration
Administration of Abciximab may result in human anti-chimeric antibody (HACA) formation that could potentially cause allergic or hypersensitivity reactions (includin g anaphylaxis), thrombocytopenia or diminished benefit upon readministration of Abciximab. In the EPIC, EPILOG, and CAPTURE trials, positive HACA responses occurred in approximately 5.8% of the Abciximab-treated patients. There was no excess of hypersensitivity or allergic reactions related to Abciximab treatment. Readministration of Abciximab to 29 healthy volunteers who had not developed a HACA response after first administration has not led to any change in Abciximab pharmacokinetics or to any reduction in antiplatelet potency. However, results in this small group of patients suggest that the incidence of HACA response may be increased after readministration. Readministration to patients who have developed a positive HACA response after initial administration has not been evaluated in clinical trials.
Allergic Reactions
Anaphylaxis has not been reported for Abciximab-treated patients in any of the Phase 3 clinical trials. However, anaphylaxis may occur. If it does, administration of Abciximab should be immediately stopped and standard appropriate resuscitative measures should be initiated.
Drug Interactions
Although drug interactions with Abciximab have not been studied systematically, Abciximab has been administered to patients with ischemic heart disease treated concomitantly with a broad range of medications used in the treatment of angina, myocardial infarction and hypertension. These medications have included heparin, warfarin, beta-adrenergic receptor blockers, calcium channel antagonists, angiotensin converting enzyme inhibitors, intravenous and oral nitrates, and aspirin. Heparin, other anticoagulants, thrombolytics, and antiplatelet agents may be associated with an increase in bleeding. Patients with HACA titers may have allergic or hypersensitivity reactions when treated with other diagnostic or therapeutic monoclonal antibodies.
Carcinogenesis, Mutagenesis and Impairment of Fertility
In vitro and in vivo mutagenicity studies have not demonstrated any mutagenic effect. Long-term studies in animals have not been performed to evaluate the carcinogenic potential or effects on fertility in male or female animals.
Pregnancy Category C
Animal reproduction studies have not been conducted with Abciximab. It is also not known whether Abciximab can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Abciximab should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk or absorbed systemically after ingestion. Because many drugs are excreted in human milk, caution should be exercised when Abciximab is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients have not been studied.
ADVERSE REACTIONS:
Bleeding
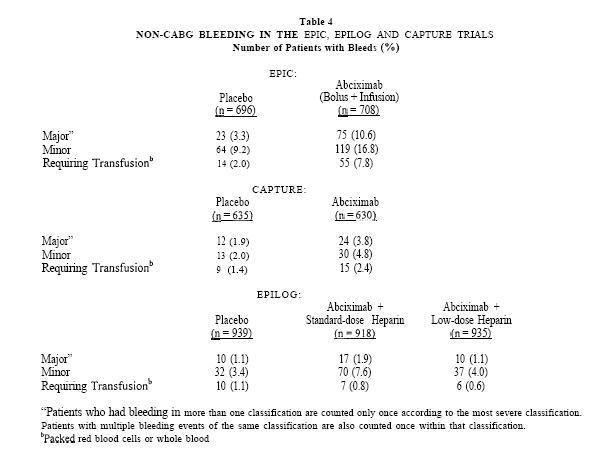
Abciximab has the potential to increase the risk of bleeding, particularly in the presence of anticoagulation, e.g. from heparin, other anticoagulants or thrombolytics. Bleeding in the Phase 3 trials was classified as major, minor or insignificant by the criteria of the Thrombolysis in Myocardial Infarction study group(7). Major bleeding events were defined as either an intracranial hemorrhage or a decrease in hemoglobin greater than 5 g/dL. Minor bleeding events included spontaneous gross hematuria, spontaneous hematemesis, observed blood loss with a hemoglobin decrease of more than 3 g/dL, or a decrease in hemoglobin of at least 4 g/dL without an identified bleeding site. Insignificant bleeding events were defined as a decrease in hemoglobin of less than 3 g/dL or a decrease in hemoglobin between 3-4 g/dL without observed bleeding. In patients who received transfusions, the number of units of blood lost was estimated through an adaptation of the method of Landefeld, et al.(s). In the EPIC trial, in which a non-weight-adjusted, standard heparin dose regimen was used, the most common complication during Abciximab therapy was bleeding during the first 36 hours. The incidences of major bleeding, minor bleeding and transfusion of blood products were significantly increased. Approximately 70% of Abciximab-treated patients with major bleeding had bleeding at the arterial access site in the groin. Abciximabtreated patients also had a higher incidence of major bleeding events from gastrointestinal, genitourinary, retroperitoneal, and other sites. Bleeding rates were reduced in the CAPTURE trial, and further reduced in the EPILOG trial by use of modified dosing regimens and specific patient management techniques. In EPILOG, using the heparin and Abciximab dosing, sheath removal and arterial access site guidelines described under PRECAUTIONS, the incidence of major bleeding in patients treated with Abciximab and low-dose, weight-adjusted heparin was not significantly different from that in patients receiving placebo. Subgroup analyses in the EPIC and CAPTURE trials showed that non-Q&G major bleeding was more common in Abciximab patients weighing < 75 kg. In the EPILOG trial which used weight-adjusted heparin dosing, the non-CABG major bleeding rates for Abciximab-treated patients did not differ substantially by weight subgroup. Although data are limited, Abciximab treatment was not associated with excess major bleeding in patients who underwent CABG surgery. (The range among all treatment arms was 3-5% in EPIC and l-2% in the CAPTURE and EPILOG trials.) Some patients with prolonged bleeding times received platelet transfusions to correct the bleeding time prior to surgery. (See PRECAUTIONS: Restoration of Platelet Function.) The rates of major bleeding, minor bleeding and bleeding events requiring transfusions in the EPIC, CAPTURE and EPILOG trials are shown in Table 4. The rates of insignificant bleeding events are not included in Table 4.
Intracranial Hemorrhage and Stroke
The total incidence of intracranial hemorrhage and non-hemorrhagic stroke across all three trials was not significantly different, 7/2225 for placebo patients and 10/3 112 for Abciximab treated patients. The incidence of intracranial hemorrhage was 3/222.5 for placebo patients and 6/3 112 for Abciximab patients.
Thrombocytopenia
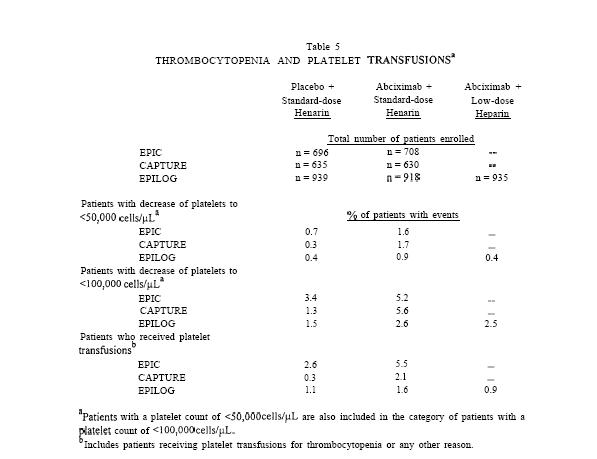
In the clinical trials, patients treated with Abciximab were more likely than patients treated with placebo to experience decreases in platelet counts. The rates of thrombocytopenia and transfusions were lower in the subsequent CAPTURE and EPILOG trials (Table 5).
Other Adverse Reactions
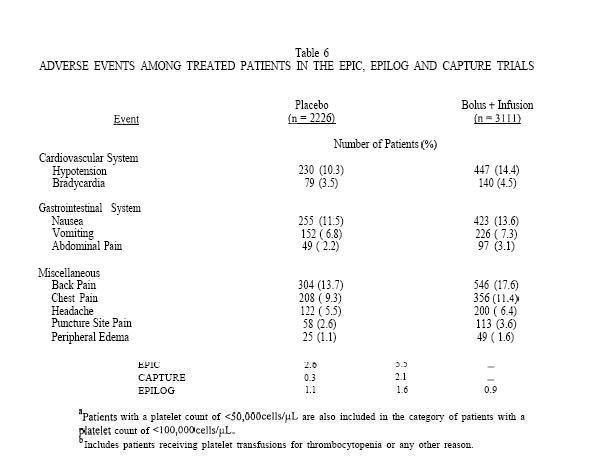
Table 6 shows adverse events other than bleeding and thrombocytopenia from the combined EPIC, EPILOG and CAPTURE trials which occurred in patients in the bolus plus infusion arm at an incidence of more than 0.5% higher than in those treated with placebo.
The following additional adverse events from the EPIC, EPILOG and CAPTURE trials were reported by investigators for patients treated with a bolus plus infusion of Abciximab at incidences which were less than 0.5% higher than for patients in the placebo arm.
Cardiovascular System-ventricular tachycardia (1.4%), pseudoaneurysm (0.8%), palpitation (0.5%), arteriovenous fistula (0.4%), incomplete AV block (0.3%), nodal arrhythmia (0.2%), complete AV block (O.l%), embolism (limb)(O.l%); thrombophlebitis (0.1%);
Gastrointestinal System Ayspepsia (2.1%), diarrhea (1 . l%), ileus (O.l%), gastroesophogeal reflux (0.1%);
Hemic and Lymphatic System-anemia (1.3%), leukocytosis (OS%), petechiae (0.2%);
Nervous System-dizziness (2.9%), anxiety (1.7%), abnormal thinking (1.3%), agitation (0.7%), hypesthesia (0.6%), confusion (0.5%) muscle contractions (0.4%), coma (0.2%), hypertonia (O.Z%), diplopia (0.1%);
Respiratory System-pneumonia (0.4%), rales (0.4%), pleural effusion (0.3%), bronchitis (0.3%) bronchospasm (0.3%), pleurisy (0.2%), pulmonary embolism (0.2%), rhonchi (0.1%);
MusculoskeletaI System-myalgia (0.2%);
Urogenital System-urinary retention (0.7%), dysuria (0.4%), abnormal renal function (0.4%), frequent micturition (O.l%), cystalgia (0. I%), urinary incontinence (0. I %), prostatitis (O.l”h);
Miscellaneous-pain (5.4%), sweating increased (1 .O%), asthenia (0.7%) incisional pain (0.6%), pruritus (0.5%) abnormal vision (0.3%), edema (0.3%), wound (0.2%), abscess (0.2%), cellulitis (0.2%), peripheral coldness (0.2%), injection site pain (O.l%), dry mouth (O.l%), pallor (O.l%), diabetes mellitus (O.l%), hyperkalemia (O.l%>, enlarged abdomen (0. I%), bullous eruption (0. 1%), inflammation (0. l%), drug toxicity (0.1%).
OVERDOSAGE:
There has been no experience of overdosage in human clinical trials.
DOSAGE AND ADMINISTRATION:
The safety and efficacy of Abciximab have only been investigated with concomitant administration of heparin and aspirin as described in CLINICAL STUDIES. In patients with failed percutaneous coronary interventions, the continuous infusion of Abciximab should be stopped because there is no evidence for Abciximab efficacy in that setting. In the event of serious bleeding that cannot be controlled by compression, Abciximab and heparin should be discontinued immediately. The recommended dosage of Abciximab in adults is a 0.25 mgkg intravenous bolus administered IO-60 minutes before the start of percutaneous coronary intervention, followed by a continuous intravenous infusion of 0.125 pgkg/min (to a maximum of 10 pg/min) for 12 hours. Patients with unstable angina not responding to conventional medical therapy and who are planned to undergo percutaneous coronary intervention within 24 hours may be treated with an Abciximab 0.25 mgkg intravenous bolus followed by an 18 to 24-hour intravenous infusion of 10 pg/min, concluding one hour after the percutaneous coronary intervention.
Instructions for Administration
- Parenteral drug products should be inspected visually for particulate matter prior to administration. Preparations of Abciximab containing visibly opaque particles should NOT be used.
- Hypersensitivity reactions should be anticipated whenever protein solutions such as Abciximab are administered. Epinephrine, dopamine, theophylline, antihistamines and corticosteroids should be available for immediate use. If symptoms of an allergic reaction or anaphylaxis appear, the infusion should be stopped and appropriate treatment given.
- As with all parenteral drug products, aseptic procedures should be used during the administration of Abciximab.
- Withdraw the necessary amount of Abciximab for bolus injection into a syringe. Filter the bolus injection using a sterile, non-pyrogenic, low protein-binding 0.2 or 0.22 pm filter (Millipore SLGV025LS or equivalent).
- Withdraw the necessary amount of Abciximab for the continuous infusion into a syringe. Inject into an appropriate container of sterile 0.9% saline or 5% dextrose and infuse at the calculated rate via a continuous infusion pump. The continuous infusion should be filtered either upon admixture using a sterile, non-pyrogenic, low protein-binding 0.2 or 0.22 pm syringe filter (Millipore SLGV025LS or equivalent) or upon administration using an in-line, sterile, non-pyrogenic, low protein-binding 0.2 or 0.22 pm filter (Abbott #4524 or equivalent). Discard the unused portion at the end of the infusion.
- No incompatibilities have been shown with intravenous infusion fluids or commonly used cardiovascular drugs. Nevertheless, Abciximab should be administered in a separate intravenous line whenever possible and not mixed with other medications.
- No incompatibilities have been observed with glass bottles or polyvinyl chloride bags and administration sets.
HOW SUPPLIED:
Abciximab (ReoPro@) 2 mg/mL is supplied in 5 mL vials containing 10 mg (NDC 0002-7 140-O 1). Vials should be stored at 2 to 8°C (36 to 46’F). Do not freeze. DO not shake. Do not use beyond the expiration date. Discard any unused portion left in the vial.
REFERENCES:
- Tcheng J, Ellis SG, George BS. Pharmacodynamics of chimeric glycoprotein IIb/IIIa integrin antiplatelet antibody Fab 7E3 in high risk coronary angioplasty. Circulation; 1994;90: 1757- 1764.
- Simoons ML, de Boer MJ, van der Brand MJBM, et al. Randomized trial of a GPIIb/IIIa platelet receptor blocker in refractory unstable angina. Circulation;1994;89:596-603.
- EPIC Investigators. Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor b in high-risk coronary angioplasty. N Engl J Med 1994;330:956-96 1.
- Topol EJ, Califf RM, Weisman HF, et al. Randomized trial of coronary intervention with antibody against platelet IIb/IIIa integrin for reduction of clinical restenosis: results at six months. Lancet 1994:343:88 l-886.
- EPILOG Investigators. Platelet glycoprotein IIb/IIIa receptor blockade and low dose heparin during percutaneous coronary revascularization. N Eng JMed. 1997;336: 1689-1696.
- CAPTURE Investigators. Randomized placebo-controlled trial of abciximab before, during and after coronary intervention in refractory unstable angina: the CAPTURE study. Lancet 1997; 349; 1429- 1435.
- Rao, AK, Pratt C, Berke A, et al. Thrombolysis in Myocardial Infarction (TIMI) Trial - Phase I: Hemorrhagic manifestations and changes in plasma fibrinogen and the fibrinolytic system in patients treated with recombinant tissue plasminogen activator and streptokinase. J Am Coll Cardiol 1988;ll: l-l 1.
- Landefeld, CS, Cook EF, Flatley M, et al. Identification and preliminary validation of predictors of major bleeding in hospitalized patients starting anticoagulant therapy. Am J Med. 1987;82:703-7 13.
Manufactured by: Centocor B.V. Leiden, The Netherlands U.S. License Number: 1178 Distributed by: Eli Lilly and Company Indianapolis, IN 46285 Revision Date: November 4, 1997