Vibrio cholerae
| Vibrio cholerae | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
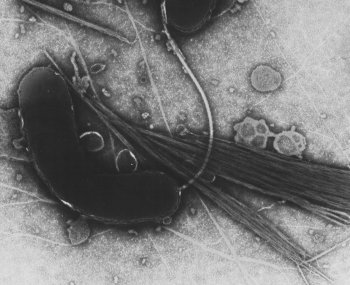 TEM image
| ||||||||||||||
| Scientific classification | ||||||||||||||
| ||||||||||||||
| Binomial name | ||||||||||||||
| Vibrio cholerae Pacini 1854 |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Vibrio cholerae is a gram negative bacterium with a curved-rod shape that causes cholera in humans.[1] V. cholerae and other species of the genus Vibrio belong to the gamma subdivision of the Proteobacteria. There are two major strains of V. cholerae, classic and El Tor, and numerous other serogroups.
V. cholerae was first isolated as the cause of cholera by Italian anatomist Filippo Pacini in 1854, but his discovery was not widely known until Robert Koch, working independently thirty years later, publicized the knowledge and the means of fighting the disease.
Habitat
V. cholerae occurs naturally in the plankton of fresh, brackish, and salt water, attached primarily to copepods in the zooplankton. Coastal cholera outbreaks typically follow zooplankton blooms. This makes cholera a typical zoonosis.
Pathogenesis
V. cholerae colonizes the gastrointestinal tract, where it adheres to villous absorptive cells via pili, and secretes a binary toxin, called cholera toxin (CT). The two CT subunits are named A and B, and are synthesised in a 1:5 ratio. B subunits bind and internalize A subunits, which are processed to A1. The A1 form catalyses ADP ribosylation from NAD to the regulatory component of adenylate cyclase, thereby activating it. Increased adenylate cyclase activity increases cyclic AMP (cAMP) synthesis causing massive fluid and electrolyte efflux, resulting in diarrhea.
CT is encoded by the ctxAB genes on a specific filamentous bacteriophage. Transduction of this phage is dependent upon bacterial expression of the Toxin Coregulated Pilus (TCP), which is encoded by the V. cholerae pathogenicity island (VPI). VPI is generally only present in virulent strains and is laterally transferred. VPI was originally thought to encode a filamentous phage responsible for transfer. This theory was discredited by a study of 46 diverse V. cholerae isolates which found no evidence of VPI phage production. The generalized transduction phage CP-T1 has been shown to transduce the entire VPI which is then integrated at the same chromosomal location. Also, VPI has been shown to excise and circularize to produce pVPI via a specialised mechanism involving VPI-encoded recombinases. It is not known whether pVPI is involved in CP-T1 transduction or if it is perhaps a component of an alternative VPI mobilization mechanism.
Additionally, it produces two different proteases called chitinase and mucinase. Chitinase is responsible for the ability of Vibrio cholerae to enter copapods. Mucinase is a non-specific protease that assists entry into the human gastro-intestinal tract.
Finally, Vibrio cholerae produces what is called a ZOT toxin, termed as "Zona Occludans Toxin". This toxin specifically attacks the zona occludans or "tight" junctions joining epithelial cells.
Differential diagnosis
Vibrio cholerae infection must be differentiated from other causes of viral, bacterial, and parasitic gastroentritis.
| Organism | Age predilection | Travel History | Incubation Size (cell) | Incubation Time | History and Symptoms | Diarrhea type8 | Food source | Specific consideration | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fever | N/V | Cramping Abd Pain | Small Bowel | Large Bowel | Inflammatory | Non-inflammatory | |||||||||
| Viral | Rotavirus | <2 y | - | <102 | <48 h | + | + | - | + | + | - | Mostly in day cares, most common in winter. | |||
| Norovirus | Any age | - | 10 -103 | 24-48 h | + | + | + | + | + | - | Most common cause of gastroenteritis, abdominal tenderness, | ||||
| Adenovirus | <2 y | - | 105 -106 | 8-10 d | + | + | + | + | + | - | No seasonality | ||||
| Astrovirus | <5 y | - | 72-96 h | + | + | + | + | + | Seafood | Mostly during winter | |||||
| Bacterial | Escherichia coli | ETEC | Any age | + | 108 -1010 | 24 h | - | + | + | + | + | - | Causes travelers diarrhea, contains heat-labile toxins (LT) and heat-stable toxins (ST) | ||
| EPEC | <1 y | - | 10† | 6-12 h | - | + | + | + | + | Raw beef and chicken | - | ||||
| EIEC | Any ages | - | 10† | 24 h | + | + | + | + | + | Hamburger meat and unpasteurized milk | Similar to shigellosis, can cause bloody diarrhea | ||||
| EHEC | Any ages | - | 10 | 3-4 d | - | + | + | + | + | Undercooked or raw hamburger (ground beef) | Known as E. coli O157:H7, can cause HUS/TTP. | ||||
| EAEC | Any ages | + | 1010 | 8-18 h | - | - | + | + | + | - | May cause prolonged or persistent diarrhea in children | ||||
| Salmonella sp. | Any ages | + | 1 | 6 to 72 h | + | + | + | + | + | Meats, poultry, eggs, milk and dairy products, fish, shrimp, spices, yeast, coconut, sauces, freshly prepared salad. | Can cause salmonellosis or typhoid fever. | ||||
| Shigella sp. | Any ages | - | 10 - 200 | 8-48 h | + | + | + | + | + | Raw foods, for example, lettuce, salads (potato, tuna, shrimp, macaroni, and chicken) | Some strains produce enterotoxin and Shiga toxin similar to those produced by E. coli O157:H7 | ||||
| Campylobacter sp. | <5 y, 15-29 y | - | 104 | 2-5 d | + | + | + | + | + | Undercooked poultry products, unpasteurized milk and cheeses made from unpasteurized milk, vegetables, seafood and contaminated water. | May cause bacteremia, Guillain-Barré syndrome (GBS), hemolytic uremic syndrome (HUS) and recurrent colitis | ||||
| Yersinia enterocolitica | <10 y | - | 104 -106 | 1-11 d | + | + | + | + | + | Meats (pork, beef, lamb, etc.), oysters, fish, crabs, and raw milk. | May cause reactive arthritis; glomerulonephritis; endocarditis; erythema nodosum.
can mimic appendicitis and mesenteric lymphadenitis. | ||||
| Clostridium perfringens | Any ages | > 106 | 16 h | - | - | + | + | + | Meats (especially beef and poultry), meat-containing products (e.g., gravies and stews), and Mexican foods. | Can survive high heat, | |||||
| Vibrio cholerae | Any ages | - | 106-1010 | 24-48 h | - | + | + | + | + | Seafoods, including molluscan shellfish (oysters, mussels, and clams), crab, lobster, shrimp, squid, and finfish. | Hypotension, tachycardia, decreased skin turgor. Rice-water stools | ||||
| Parasites | Protozoa | Giardia lamblia | 2-5 y | + | 1 cyst | 1-2 we | - | - | + | + | + | Contaminated water | May cause malabsorption syndrome and severe weight loss | ||
| Entamoeba histolytica | 4-11 y | + | <10 cysts | 2-4 we | - | + | + | + | + | Contaminated water and raw foods | May cause intestinal amebiasis and amebic liver abscess | ||||
| Cryptosporidium parvum | Any ages | - | 10-100 oocysts | 7-10 d | + | + | + | + | + | Juices and milk | May cause copious diarrhea and dehydration in patients with AIDS especially with 180 > CD4 | ||||
| Cyclospora cayetanensis | Any ages | + | 10-100 oocysts | 7-10 d | - | + | + | + | + | Fresh produce, such as raspberries, basil, and several varieties of lettuce. | More common in rainy areas | ||||
| Helminths | Trichinella spp | Any ages | - | Two viable larvae (male and female) | 1-4 we | - | + | + | + | + | Undercooked meats | More common in hunters or people who eat traditionally uncooked meats | |||
| Taenia spp | Any ages | - | 1 larva or egg | 2-4 m | - | + | + | + | + | Undercooked beef and pork | Neurocysticercosis: Cysts located in the brain may be asymptomatic or seizures, increased intracranial pressure, headache. | ||||
| Diphyllobothrium latum | Any ages | - | 1 larva | 15 d | - | - | - | + | + | Raw or undercooked fish. | May cause vitamin B12 deficiency | ||||
8Small bowel diarrhea: watery, voluminous with less than 5 WBC/high power field
Large bowel diarrhea: Mucousy and/or bloody with less volume and more than 10 WBC/high power field
† It could be as high as 1000 based on patient's immunity system.
The table below summarizes the findings that differentiate inflammatory causes of chronic diarrhea[2][3][4][5][5]
| Cause | History | Laboratory findings | Diagnosis | Treatment |
|---|---|---|---|---|
| Diverticulitis |
|
|
Abdominal CT scan with oral and intravenous (IV) contrast | bowel rest, IV fluid resuscitation, and broad-spectrum antimicrobial therapy which covers anaerobic bacteria and gram-negative rods |
| Ulcerative colitis |
|
|
Endoscopy | Induction of remission with mesalamine and corticosteroids followed by the administration of sulfasalazine and 6-Mercaptopurine depending on the severity of the disease. |
| Entamoeba histolytica |
|
cysts shed with the stool | detects ameba DNA in feces | Amebic dysentery
Luminal amebicides for E. histolytica in the colon:
For amebic liver abscess:
|
References
- ↑ Ryan KJ; Ray CG (editors) (2004). Sherris Medical Microbiology (4th ed. ed.). McGraw Hill. ISBN 0838585299.
- ↑ Konvolinka CW (1994). "Acute diverticulitis under age forty". Am J Surg. 167 (6): 562–5. PMID 8209928.
- ↑ Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR; et al. (2005). "Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology". Can J Gastroenterol. 19 Suppl A: 5A–36A. PMID 16151544.
- ↑ Satsangi J, Silverberg MS, Vermeire S, Colombel JF (2006). "The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications". Gut. 55 (6): 749–53. doi:10.1136/gut.2005.082909. PMC 1856208. PMID 16698746.
- ↑ 5.0 5.1 Haque R, Huston CD, Hughes M, Houpt E, Petri WA (2003). "Amebiasis". N Engl J Med. 348 (16): 1565–73. doi:10.1056/NEJMra022710. PMID 12700377.
External links
bn:ভিব্রিও কলেরী
de:Vibrio cholerae
it:Vibrio cholerae
he:Vibrio cholerae
nl:Vibrio cholerae
[[sk:Vibrio cholerae]