Topiramate: Difference between revisions
No edit summary |
No edit summary |
||
| Line 56: | Line 56: | ||
* Hyperammonemia and encephalopathy: Patients with inborn errors of metabolism or reduced mitochondrial activity may have an increased risk of hyperammonemia. Measure ammonia if encephalopathic symptoms occur | * Hyperammonemia and encephalopathy: Patients with inborn errors of metabolism or reduced mitochondrial activity may have an increased risk of hyperammonemia. Measure ammonia if encephalopathic symptoms occur | ||
* Kidney stones: Avoid use with other carbonic anhydrase inhibitors, other drugs causing metabolic acidosis, or in patients on a ketogenic diet | * Kidney stones: Avoid use with other carbonic anhydrase inhibitors, other drugs causing metabolic acidosis, or in patients on a ketogenic diet | ||
* Hypothermia: Reported with concomitant valproic acid use | * Hypothermia: Reported with concomitant valproic acid use | ||
|drugInteractions=*Oral Contraceptives | |||
:*The possibility of decreased contraceptive efficacy and increased breakthrough bleeding should be considered in patients taking combination oral contraceptive products with QUDEXY XR. Patients taking estrogen-containing contraceptives should be asked to report any change in their bleeding patterns. Contraceptive efficacy can be decreased even in the absence of breakthrough bleeding [see Clinical Pharmacology | |||
*Antiepileptic Drugs | |||
:*Concomitant administration of phenytoin or carbamazepine with topiramate decreased plasma concentrations of topiramate | |||
:*Concomitant administration of valproic acid and topiramate has been associated with hyperammonemia with and without encephalopathy. Concomitant administration of topiramate with valproic acid has also been associated with hypothermia (with and without hyperammonemia) in patients who have tolerated either drug alone. It may be prudent to examine blood ammonia levels in patients in whom the onset of hypothermia has been reported | |||
:*Numerous AEDs are substrates of the CYP enzyme system. In vitro studies indicate that topiramate does not inhibit enzyme activity for CYP1A2, CYP2A6, CYP2B6, CYP2C9, CYP2D6, CYP2E1, and CYP3A4/5 isozymes. In vitro studies indicate that immediate-release topiramate is a mild inhibitor of CYP2C19 and a mild inducer of CYP3A4. The same drug interactions can be expected with the use of QUDEXY XR. | |||
*CNS Depressants and Alcohol | |||
* | :*Topiramate is a CNS depressant. Concomitant administration of topiramate with other CNS depressant drugs or alcohol can result in significant CNS depression. Concomitant use of alcohol should be avoided [see Warnings and Precautions (5.13) and Clinical Pharmacology (12.3)]. | ||
* Other Carbonic Anhydrase Inhibitors | |||
:*Concomitant use of topiramate, a carbonic anhydrase inhibitor, with any other carbonic anhydrase inhibitor (e.g., zonisamide, acetazolamide or dichlorphenamide), may increase the severity of metabolic acidosis and may also increase the risk of kidney stone formation. Patients should be monitored for the appearance or worsening of metabolic acidosis when QUDEXY XR is given concomitantly with another carbonic anhydrase inhibitor | |||
*Metformin | |||
:*Topiramate treatment can frequently cause metabolic acidosis, a condition for which the use of metformin is contraindicated. The concomitant use of QUDEXY XR and metformin is contraindicated in patients with metabolic acidosis | |||
*Lithium | |||
:*In patients, there was an observed increase in systemic exposure of lithium following topiramate doses of up to 600 mg per day. Lithium levels should be monitored when co-administered with high-dose QUDEXY XR | |||
|useInPregnancyFDA=Increased risk of cleft lip and/or palate. Pregnancy registry available | |useInPregnancyFDA=Increased risk of cleft lip and/or palate. Pregnancy registry available | ||
|useInNursing=Caution should be exercised when administered to a nursing mother | |useInNursing=Caution should be exercised when administered to a nursing mother | ||
|useInGeri=Dosage adjustment may be necessary for elderly with impaired renal function | |useInGeri=Dosage adjustment may be necessary for elderly with impaired renal function | ||
|useInRenalImpair=(creatinine clearance less than 70 mL/min/1.73m2), one-half of the adult dose is recommended | |useInRenalImpair=(creatinine clearance less than 70 mL/min/1.73m2), one-half of the adult dose is recommended | ||
|othersTitle=Patients undergoing hemodialysis | |othersTitle=Patients undergoing hemodialysis | ||
|useInOthers=Topiramate is cleared by hemodialysis. Dosage adjustment is necessary to avoid rapid drops in topiramate plasma concentration during hemodialysis | |useInOthers=Topiramate is cleared by hemodialysis. Dosage adjustment is necessary to avoid rapid drops in topiramate plasma concentration during hemodialysis | ||
|administration=[[File:Topi dosage and admin.PNG|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |administration=[[File:Topi dosage and admin.PNG|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
|drugBox={{drugbox2 | |drugBox={{drugbox2 | ||
Revision as of 21:34, 28 May 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Pratik Bahekar, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Topiramate is a Mood Stabilizer that is FDA approved for the {{{indicationType}}} of Lennox-Gastaut syndrome (adjunct), migraine (prophylaxis), partial seizure (initial monotherapy), partial seizure(adjunct), tonic-clonic seizure, primary generalized (adjunct), tonic-clonic seizure, primary generalized (initial monotherapy). Common adverse reactions include flushing, serum bicarbonate level abnormal, loss of appetite, weight decreased, infectious disease, confusion, dizziness, impaired cognition, impaired, psychomotor performance, memory impairment, paresthesia, reduced concentration span, somnolence, feeling nervous, mood disorder, fatigue, fever.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Lennox-Gastaut syndrome
- Start 25 to 50 mg/day PO, increase dosage by 25 to 50 mg/day at 1-week intervals to maintenance dose of 200 to 400 mg/day in 2 divided doses.
- Migraine prophylaxis
- 100 mg/day PO administered in 2 divided doses; 25 mg in the evening for 1 week, 25 mg twice daily for 1 week, 25 mg in the morning and 50 mg in the evening for 1 week, and then 50 mg twice daily.
- Partial seizure, initial monotherapy
- 25 mg PO twice daily; second week 50 mg PO twice daily, third week 75 mg PO twice daily, fourth week 100 mg PO twice daily, fifth week 150 mg PO twice daily, sixth week (MAX dose) 200 mg PO
- Partial seizure; adjunct
- 25 to 50 mg/day PO; may increase dosage by 25 to 50 mg/day at 1-week intervals to the usual maintenance dose of 200 to 400 mg/day in 2 divided doses.
- Tonic-clonic seizure, primary generalized (adjunct)
- 25 to 50 mg/day ORALLY; may increase dosage by 25 to 50 mg/day at 1-week intervals to the usual maintenance dose of 400 mg/day in 2 divided doses.
- Tonic-clonic seizure, primary generalized (initial monotherapy).
- 25 mg PO twice daily (morning and evening); second week, 50 mg PO twice daily; third week, 75 mg PO twice daily; fourth week, 100 mg PO twice daily; fifth week, 150 mg PO twice daily; sixth week (MAX dose), 200 mg PO twice daily
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Topiramate in adult patients.
Non–Guideline-Supported Use
- Alcoholism
- Diabetes mellitus type 2 in obese (adjunct)
- Eating disorder.
- Essential tremor
- Obesity
There is limited information about Off-Label Non–Guideline-Supported Use of Topiramate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Lennox-Gastaut syndrome
- 2 years or older for opamax(R) and Qudexy(TM) XR,
- 6 years or older for Trokendi XR(TM)
- Migraine prophylaxis
- 12 years or older for Topamax(R) only
- Partial seizure, initial monotherapy
- 2 years or older for Qudexy XR (TM)
- 10 years or older for Trokendi XR(TM),
- Partial seizure; adjunct
- 2 years or older for Topamax(R) and Qudexy(TM) XR,
- 6 years or older Trokendi XR(TM)
- Tonic-clonic seizure, primary generalized (adjunct)
- 2 years or older for Topamax(R) and Qudexy(TM) XR
- 6 years or older for Trokendi XR(TM)
- Tonic-clonic seizure, primary generalized (initial monotherapy)
- 2 years or older for Topamax(R)
- 10 years or older for Trokendi XR(TM) and Qudexy(TM) XR
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Topiramate in pediatric patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Topiramate in pediatric patients.
Contraindications
There is limited information regarding Topiramate Contraindications in the drug label.
Warnings
- Acute myopia and secondary angle closure glaucoma: Untreated elevated intraocular pressure can lead to permanent visual loss. Discontinue QUDEXY XR if it occurs
- Visual field defects: These have been reported independent of elevated intraocular pressure. Consider discontinuation of QUDEXY XR
- Oligohydrosis and hyperthermia: Monitor decreased sweating and increased body temperature, especially in pediatric patients
- Metabolic acidosis: Measure baseline and periodic measurement of serum bicarbonate. Consider dose reduction or discontinuation of QUDEXY XR if clinically appropriate
- Suicidal behavior and ideation: Antiepileptic drugs increase the risk of suicidal behavior or ideation
- Cognitive/neuropsychiatric: QUDEXY XR may cause cognitive dysfunction. Use caution when operating machinery including automobiles. Depression and mood problems may occur
- Fetal toxicity: Topiramate use during pregnancy can cause cleft lip and/or palate (5.7)
- Withdrawal of AEDs: Withdrawal of QUDEXY XR should be done gradually (5.8)
- Hyperammonemia and encephalopathy: Patients with inborn errors of metabolism or reduced mitochondrial activity may have an increased risk of hyperammonemia. Measure ammonia if encephalopathic symptoms occur
- Kidney stones: Avoid use with other carbonic anhydrase inhibitors, other drugs causing metabolic acidosis, or in patients on a ketogenic diet
- Hypothermia: Reported with concomitant valproic acid use
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Topiramate Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Topiramate Postmarketing Experience in the drug label.
Drug Interactions
- Oral Contraceptives
- The possibility of decreased contraceptive efficacy and increased breakthrough bleeding should be considered in patients taking combination oral contraceptive products with QUDEXY XR. Patients taking estrogen-containing contraceptives should be asked to report any change in their bleeding patterns. Contraceptive efficacy can be decreased even in the absence of breakthrough bleeding [see Clinical Pharmacology
- Antiepileptic Drugs
- Concomitant administration of phenytoin or carbamazepine with topiramate decreased plasma concentrations of topiramate
- Concomitant administration of valproic acid and topiramate has been associated with hyperammonemia with and without encephalopathy. Concomitant administration of topiramate with valproic acid has also been associated with hypothermia (with and without hyperammonemia) in patients who have tolerated either drug alone. It may be prudent to examine blood ammonia levels in patients in whom the onset of hypothermia has been reported
- Numerous AEDs are substrates of the CYP enzyme system. In vitro studies indicate that topiramate does not inhibit enzyme activity for CYP1A2, CYP2A6, CYP2B6, CYP2C9, CYP2D6, CYP2E1, and CYP3A4/5 isozymes. In vitro studies indicate that immediate-release topiramate is a mild inhibitor of CYP2C19 and a mild inducer of CYP3A4. The same drug interactions can be expected with the use of QUDEXY XR.
- CNS Depressants and Alcohol
- Topiramate is a CNS depressant. Concomitant administration of topiramate with other CNS depressant drugs or alcohol can result in significant CNS depression. Concomitant use of alcohol should be avoided [see Warnings and Precautions (5.13) and Clinical Pharmacology (12.3)].
- Other Carbonic Anhydrase Inhibitors
- Concomitant use of topiramate, a carbonic anhydrase inhibitor, with any other carbonic anhydrase inhibitor (e.g., zonisamide, acetazolamide or dichlorphenamide), may increase the severity of metabolic acidosis and may also increase the risk of kidney stone formation. Patients should be monitored for the appearance or worsening of metabolic acidosis when QUDEXY XR is given concomitantly with another carbonic anhydrase inhibitor
- Metformin
- Topiramate treatment can frequently cause metabolic acidosis, a condition for which the use of metformin is contraindicated. The concomitant use of QUDEXY XR and metformin is contraindicated in patients with metabolic acidosis
- Lithium
- In patients, there was an observed increase in systemic exposure of lithium following topiramate doses of up to 600 mg per day. Lithium levels should be monitored when co-administered with high-dose QUDEXY XR
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
Increased risk of cleft lip and/or palate. Pregnancy registry available
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Topiramate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Topiramate during labor and delivery.
Nursing Mothers
Caution should be exercised when administered to a nursing mother
Pediatric Use
There is no FDA guidance on the use of Topiramate in pediatric settings.
Geriatic Use
Dosage adjustment may be necessary for elderly with impaired renal function
Gender
There is no FDA guidance on the use of Topiramate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Topiramate with respect to specific racial populations.
Renal Impairment
(creatinine clearance less than 70 mL/min/1.73m2), one-half of the adult dose is recommended
Hepatic Impairment
There is no FDA guidance on the use of Topiramate in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Topiramate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Topiramate in patients who are immunocompromised.
Patients undergoing hemodialysis
Topiramate is cleared by hemodialysis. Dosage adjustment is necessary to avoid rapid drops in topiramate plasma concentration during hemodialysis
Administration and Monitoring
Administration

Monitoring
There is limited information regarding Topiramate Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Topiramate and IV administrations.
Overdosage
There is limited information regarding Topiramate overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
| |
| |
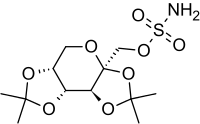
Topiramate
| |
| Systematic (IUPAC) name | |
| 2,3:4,5-Bis-O-(1-methylethylidene)-beta-D-fructopyranose sulfamate | |
| Identifiers | |
| CAS number | |
| ATC code | N03 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 339.363 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 80% |
| Protein binding | 13-17%; 15-41% |
| Metabolism | Hepatic (20-30%) |
| Half life | 19-25 hours |
| Excretion | Urine (70-80%) |
| Therapeutic considerations | |
| Licence data |
|
| Pregnancy cat. | |
| Legal status |
Prescription Only (S4)(AU) ?(CA) POM(UK) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | Oral |
Mechanism of Action
There is limited information regarding Topiramate Mechanism of Action in the drug label.
Structure
There is limited information regarding Topiramate Structure in the drug label.
Pharmacodynamics
There is limited information regarding Topiramate Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Topiramate Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Topiramate Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Topiramate Clinical Studies in the drug label.
How Supplied
There is limited information regarding Topiramate How Supplied in the drug label.
Storage
There is limited information regarding Topiramate Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Topiramate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Topiramate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Topiramate Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Topiramate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Topiramate Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Topiramate Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.