Tisagenlecleucel
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Yashasvi Aryaputra[2];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
CYTOKINE RELEASE SYNDROME AND NEUROLOGICAL TOXICITIES
See full prescribing information for complete Boxed Warning.
|
Overview
Tisagenlecleucel is a Acetylcholine release inhibitor, Adrenergic receptor agonist that is FDA approved for the (type of indication of drug) of a list of indications, separated by commas.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include a list of adverse reactions, separated by commas..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
CONTRAINDICATIONS
Warnings
|
CYTOKINE RELEASE SYNDROME AND NEUROLOGICAL TOXICITIES
See full prescribing information for complete Boxed Warning.
|
Conidition 1
(Description)
Conidition 2
(Description)
Conidition 3
(Description)
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Condition 2
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
(Description)
Drug Interactions
- Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
(Description)
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Tisagenlecleucel in women who are pregnant.
Labor and Delivery
(Description)
Nursing Mothers
(Description)g
Pediatric Use
(Description)
Geriatic Use
(Description)
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
There is limited information regarding the compatibility of Tisagenlecleucel and IV administrations.
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
Tisagenlecleucel
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
(Description)
Structure
(Description with picture)
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
Condition 1
(Description)
Condition 2
(Description)
Condition 3
(Description)
How Supplied
- KYMRIAH is supplied as a frozen suspension of genetically modified autologous T cells in an infusion bag(s) labeled for the specific recipient. KYMRIAH is shipped directly to the cell lab associated with the infusion center in a liquid nitrogen Dewar. Product and patient-specific labels are located inside the Dewar.
- Ped ALL: NDC 0078-0846-19
- DLBCL: NDC 0078-0958-19
Storage
- Confirm patient identity upon receipt.
- Store infusion bag(s) in the vapor phase of liquid nitrogen (less than or equal to minus 120°C) in a temperature-monitored system.
- Use closed, break-proof, leak-proof containers when transporting infusion bags within the facility.
- Thaw KYMRIAH prior to infusion.
Images
Drug Images
{{#ask: Page Name::Tisagenlecleucel |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Tisagenlecleucel |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
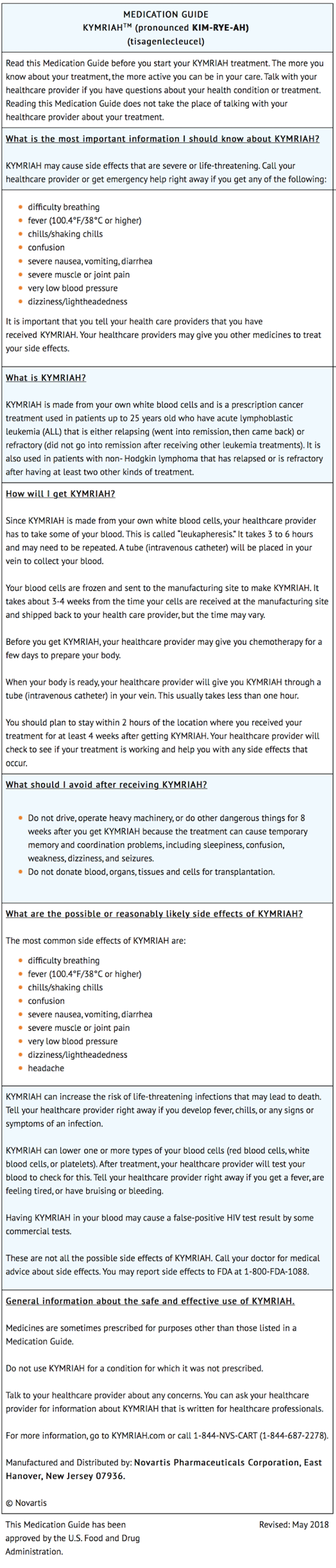
- Ensure that patients understand the risk of manufacturing failure. This has been reported in up to 9% of manufacturing attempts. In case of a manufacturing failure, a second manufacturing of KYMRIAH may be attempted. In addition, while the patient awaits the product, additional chemotherapy (not the lymphodepletion) may be necessary and may increase the risk of adverse events during the pre-infusion period.
- Prior to infusion, advise patients of the following risks:
- Cytokine Release Syndrome (CRS) -- Report signs and symptoms of CRS (high fever, difficulty breathing, chills/shaking chills, severe nausea, severe vomiting, severe diarrhea, severe muscle pain, severe joint pain, very low blood pressure, or dizziness/lightheadedness) to their healthcare professional.
- Neurological Toxicities -- Report altered or decreased consciousness, delirium, confusion, agitation, seizures, difficulty speaking and understanding, or loss of balance to their healthcare professional.
- Serious Infections -- KYMRIAH may cause serious infections. Advise patients that they will be screened for HBV, HCV, and HIV before collection of cells.
- Hypogammaglobulinemia -- Patients may need to receive immunoglobulin replacement for an indefinite amount of time following treatment with KYMRIAH. Patients should tell their physician about their treatment with KYMRIAH before receiving a live virus vaccine.
- Driving and Engaging in Hazardous Occupations -- Patients should refrain from driving and engaging in hazardous occupations or activities, such as operating heavy or potentially dangerous machinery, for at least 8 weeks after treatment.
- Prolonged Cytopenia -- Patient may exhibit signs or symptoms associated with bone marrow suppression (i.e., neutropenia, thrombocytopenia and anemia) for several weeks following lymphodepleting chemotherapy and KYMRIAH.
- Patients should be instructed to contact Novartis Pharmaceuticals Corporation at 1-844-4KYMRIAH if they get secondary malignancies.
Precautions with Alcohol
Alcohol-Tisagenlecleucel interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Kymriah
Look-Alike Drug Names
There is limited information regarding Tisagenlecleucel Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.