Tisagenlecleucel: Difference between revisions
No edit summary |
No edit summary |
||
| Line 131: | Line 131: | ||
(Description) | (Description) | ||
|clinicalTrials= | |clinicalTrials=*Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | ||
*The safety data described in the WARNINGS AND PRECAUTIONS and in this section reflect exposure to KYMRIAH in two non-randomized, single-arm studies in which 68 pediatric and young adult patients with relapsed/refractory (r/r) B-cell ALL (ELIANA Study) and 106 adults with r/r diffuse large B-cell lymphoma (JULIET Study) received a single dose of CAR-positive viable T cells. | |||
''Pediatric and Young Adult r/r B-cell Acute Lymphoblastic Leukemia (ALL) (up to 25 years of age)'' | |||
*Based on a recommended dose which was weight-based, all 68 patients in the ELIANA study (Study 1) received a single intravenous dose of KYMRIAH [see Clinical Studies (14.1)]. The most common adverse reactions (> 20%) were cytokine release syndrome (79%), hypogammaglobulinemia (43%), infections-pathogen unspecified (41%), pyrexia (40%), decreased appetite (37%), headache (37%), encephalopathy (34%), hypotension (31%), bleeding episodes (31%), tachycardia (26%), nausea (26%), diarrhea (26%), vomiting (26%), viral infectious disorders (26%), hypoxia (24%), fatigue (25%), acute kidney injury (24%), edema (21%), cough (21%), and delirium (21%). | |||
*The adverse reactions with greater or equal to 10% incidence for any Grade are summarized in Table 2. | |||
: | [[image:Tisagenlecleucel_Adverse_Reactions_Tables_1_and_2.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | ||
Laboratory Abnormalities | |||
*Selected laboratory abnormalities worsening from baseline Grade 0-2 to Grade 3-4 are shown in Table 3. | |||
[[image:Tisagenlecleucel_Adverse_Reactions_Table_3.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | |||
*All patients experienced neutropenia, anemia and thrombocytopenia. See Table 4 for the incidences of ≥ Grade 3 prolonged thrombocytopenia and prolonged neutropenia in responding patients. | |||
[[image:Tisagenlecleucel_Adverse_Reactions_Table_4.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | |||
''Adult r/r Diffuse Large B-cell Lymphoma (DLBCL)'' | |||
*In the JULIET study (Study 2) 106 adults with r/r DLBCL received a single intravenous dose of KYMRIAH. The most common adverse reactions (incidence > 20%) were cytokine release syndrome, infections-pathogen unspecified, diarrhea, nausea, pyrexia, fatigue, hypotension, edema and headache. | |||
== | *The study population characteristics were: median age of 56 years (range: 22 to 76 years), 79% DLBCL; a median of 3 prior lines of therapy (range: 1-6), 49% had a prior autologous hematopoietic stem cell transplantation, and 33% had received prior radiation therapy. Ninety-nine patients (93%) received lymphodepleting chemotherapy prior to KYMRIAH, that included fludarabine (n = 77) or bendamustine (n = 22). | ||
*The adverse reactions with greater than or equal to 10% incidence for any Grade are summarized in Table 5 below. | |||
[[image:Tisagenlecleucel_Adverse_Reactions_Tables_5_and_6.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | |||
Laboratory Abnormalities | |||
*Selected laboratory abnormalities worsening from baseline Grade 0-2 to Grade 3-4 are shown in Table 6. | |||
: | [[image:Tisagenlecleucel_Adverse_Reactions_Table_7.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | ||
===== | =====Immunogenicity===== | ||
*In clinical studies, humoral immunogenicity of KYMRIAH was measured by determination of anti-murine CAR19 antibodies (anti-mCAR19) in serum pre- and post-administration. The majority of patients, 86% in ELIANA (Study 1) and 91.4% in JULIET (Study 2) tested positive for pre-dose anti-mCAR19 antibodies prior to KYMRIAH infusion; Treatment induced anti-mCAR19 antibodies were detected in 5% of the patients in JULIET. However, the preexisting and treatment-induced antibodies were not associated with an impact on clinical response and did not have an impact on the initial expansion and persistence of KYMRIAH. Persistence of KYMRIAH was similar between patients with positive post-infusion anti-mCAR19 antibodies compared with patients with negative post-infusion anti-mCAR19 antibodies. There is no evidence that the presence of preexisting and treatment-induced anti-mCAR19 antibodies impact the safety or effectiveness of KYMRIAH. | |||
*T cell immunogenicity responses were not observed in adult r/r DLBCL patients. | |||
|postmarketing=(Description) | |postmarketing=(Description) | ||
|drugInteractions=* | |drugInteractions=*HIV and the lentivirus used to make KYMRIAH have limited, short spans of identical genetic material (RNA). Therefore, some commercial HIV nucleic acid test (NATs) tests may yield false-positive results in patients who have received KYMRIAH. | ||
( | |||
( | |||
|useInPregnancyFDA=Risk Summary | |useInPregnancyFDA=Risk Summary | ||
Revision as of 14:22, 1 August 2018
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Yashasvi Aryaputra[2];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
CYTOKINE RELEASE SYNDROME AND NEUROLOGICAL TOXICITIES
See full prescribing information for complete Boxed Warning.
|
Overview
Tisagenlecleucel is a Acetylcholine release inhibitor, Adrenergic receptor agonist that is FDA approved for the (type of indication of drug) of a list of indications, separated by commas.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include a list of adverse reactions, separated by commas..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
CONTRAINDICATIONS
Warnings
|
CYTOKINE RELEASE SYNDROME AND NEUROLOGICAL TOXICITIES
See full prescribing information for complete Boxed Warning.
|
Conidition 1
(Description)
Conidition 2
(Description)
Conidition 3
(Description)
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The safety data described in the WARNINGS AND PRECAUTIONS and in this section reflect exposure to KYMRIAH in two non-randomized, single-arm studies in which 68 pediatric and young adult patients with relapsed/refractory (r/r) B-cell ALL (ELIANA Study) and 106 adults with r/r diffuse large B-cell lymphoma (JULIET Study) received a single dose of CAR-positive viable T cells.
Pediatric and Young Adult r/r B-cell Acute Lymphoblastic Leukemia (ALL) (up to 25 years of age)
- Based on a recommended dose which was weight-based, all 68 patients in the ELIANA study (Study 1) received a single intravenous dose of KYMRIAH [see Clinical Studies (14.1)]. The most common adverse reactions (> 20%) were cytokine release syndrome (79%), hypogammaglobulinemia (43%), infections-pathogen unspecified (41%), pyrexia (40%), decreased appetite (37%), headache (37%), encephalopathy (34%), hypotension (31%), bleeding episodes (31%), tachycardia (26%), nausea (26%), diarrhea (26%), vomiting (26%), viral infectious disorders (26%), hypoxia (24%), fatigue (25%), acute kidney injury (24%), edema (21%), cough (21%), and delirium (21%).
- The adverse reactions with greater or equal to 10% incidence for any Grade are summarized in Table 2.

Laboratory Abnormalities
- Selected laboratory abnormalities worsening from baseline Grade 0-2 to Grade 3-4 are shown in Table 3.

- All patients experienced neutropenia, anemia and thrombocytopenia. See Table 4 for the incidences of ≥ Grade 3 prolonged thrombocytopenia and prolonged neutropenia in responding patients.

Adult r/r Diffuse Large B-cell Lymphoma (DLBCL)
- In the JULIET study (Study 2) 106 adults with r/r DLBCL received a single intravenous dose of KYMRIAH. The most common adverse reactions (incidence > 20%) were cytokine release syndrome, infections-pathogen unspecified, diarrhea, nausea, pyrexia, fatigue, hypotension, edema and headache.
- The study population characteristics were: median age of 56 years (range: 22 to 76 years), 79% DLBCL; a median of 3 prior lines of therapy (range: 1-6), 49% had a prior autologous hematopoietic stem cell transplantation, and 33% had received prior radiation therapy. Ninety-nine patients (93%) received lymphodepleting chemotherapy prior to KYMRIAH, that included fludarabine (n = 77) or bendamustine (n = 22).
- The adverse reactions with greater than or equal to 10% incidence for any Grade are summarized in Table 5 below.

Laboratory Abnormalities
- Selected laboratory abnormalities worsening from baseline Grade 0-2 to Grade 3-4 are shown in Table 6.

Immunogenicity
- In clinical studies, humoral immunogenicity of KYMRIAH was measured by determination of anti-murine CAR19 antibodies (anti-mCAR19) in serum pre- and post-administration. The majority of patients, 86% in ELIANA (Study 1) and 91.4% in JULIET (Study 2) tested positive for pre-dose anti-mCAR19 antibodies prior to KYMRIAH infusion; Treatment induced anti-mCAR19 antibodies were detected in 5% of the patients in JULIET. However, the preexisting and treatment-induced antibodies were not associated with an impact on clinical response and did not have an impact on the initial expansion and persistence of KYMRIAH. Persistence of KYMRIAH was similar between patients with positive post-infusion anti-mCAR19 antibodies compared with patients with negative post-infusion anti-mCAR19 antibodies. There is no evidence that the presence of preexisting and treatment-induced anti-mCAR19 antibodies impact the safety or effectiveness of KYMRIAH.
- T cell immunogenicity responses were not observed in adult r/r DLBCL patients.
Postmarketing Experience
(Description)
Drug Interactions
- HIV and the lentivirus used to make KYMRIAH have limited, short spans of identical genetic material (RNA). Therefore, some commercial HIV nucleic acid test (NATs) tests may yield false-positive results in patients who have received KYMRIAH.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Risk Summary
- There are no available data with KYMRIAH use in pregnant women. No animal reproductive and developmental toxicity studies have been conducted with KYMRIAH to assess whether it can cause fetal harm when administered to a pregnant woman. It is not known if KYMRIAH has the potential to be transferred to the fetus. Based on the mechanism of action, if the transduced cells cross the placenta, they may cause fetal toxicity, including B-cell lymphocytopenia. Therefore, KYMRIAH is not recommended for women who are pregnant, and pregnancy after KYMRIAH administration should be discussed with the treating physician. Report pregnancies to Novartis Pharmaceuticals Corporation at 1-888-669-6682.
- In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%-4% and 15%-20%, respectively.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Tisagenlecleucel in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Tisagenlecleucel during labor and delivery.
Nursing Mothers
Risk Summary
- There is no information regarding the presence of KYMRIAH in human milk, the effect on the breastfed infant, and the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for KYMRIAH and any potential adverse effects on the breastfed infant from KYMRIAH or from the underlying maternal condition.
Pediatric Use
- The safety and efficacy of KYMRIAH have been established in pediatric patients with r/r B-cell ALL. Use of KYMRIAH is supported by a single-arm trial [see Clinical Studies (14.1)] that included 52 pediatric patients with r/r B-cell precursor ALL in the following age groups: 33 children (age 3 years to less than 12 years) and 19 adolescents (age 12 years to less than 17 years). No differences in efficacy or safety were observed between the different age subgroups or in comparison to the young adults in the trial.
- The safety and efficacy of KYMRIAH in pediatric patients with relapsed or refractory DLBCL has not been established.
Geriatic Use
- The safety and effectiveness of KYMRIAH have not been established in geriatric patients with r/r B-cell ALL. Clinical studies of KYMRIAH did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
Gender
There is no FDA guidance on the use of Tisagenlecleucel with respect to specific gender populations.
Race
There is no FDA guidance on the use of Tisagenlecleucel with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Tisagenlecleucel in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Tisagenlecleucel in patients with hepatic impairment.
Females of Reproductive Potential and Males
Pregnancy Testing
- Pregnancy status of females with reproductive potential should be verified. Sexually-active females of reproductive potential should have a pregnancy test prior to starting treatment with KYMRIAH.
Contraception
- See the prescribing information for fludarabine and cyclophosphamide for information on the need for effective contraception in patients who receive the lymphodepleting chemotherapy.
- There are insufficient exposure data to provide a recommendation concerning duration of contraception following treatment with KYMRIAH.
Infertility
- There are no data on the effect of KYMRIAH on fertility.
Immunocompromised Patients
There is no FDA guidance one the use of Tisagenlecleucel in patients who are immunocompromised.
Administration and Monitoring
Administration
Intravenous
- Match patient identity with patient identifiers on infusion bag (patient name, date of birth, and DIN or Aph ID) prior to preparation.
- A Tisagenlecleucel dose may be contained in up to 3 cryopreserved patient-specific infusion bags. Verify the number of bags received for the dose of Tisagenlecleucel with the Certificate of Analysis (CoA).
- Coordinate thaw with timing of infusion; confirm infusion time and adjust thaw start time to ensure that product is available for infusion when recipient is ready as thawed product may only be stored for up to 30 minutes at room temperature (20 to 25 degrees C). If more than 1 bag has been received for the treatment dose, thaw 1 bag at a time. Wait to thaw or infuse the next bag until it is determined that the previous bag has been safely administered.
- Do not use if bag is compromised (eg, breaks or cracks in bag).
- Place infusion bag inside a second, sterile bag to protect ports from contamination or in case of a leak.
- Thaw infusion bag in water bath or by dry thaw method at 37 degrees C until no visible ice is present.
- Do not resuspend in new media, wash, or spin down before infusing.
- Inspect thawed infusion bag for visible clumps and gently manually mix to disperse; do not infuse if clumps do not disperse.
- May be stored for up to 30 minutes at room temperature (20 to 25 degrees C) after thawing; infuse within 30 minutes of thawing.
- Prime tubing with NS prior to infusion.
- Do not administer through a leukocyte-depleting filter.
- Infuse entire contents of bag (10 to 50 mL) at 10 to 20 mL/min; adjust rate for smaller children and volumes.
- Cells from all bags must be infused to complete a single dose.
- While maintaining a closed tubing system, rinse infusion bag with 10 to 30 mL NS to ensure infusion of as many cells as possible into patient.
Monitoring
- Resolution or improvement of disease-related signs (reduction of blasts in the bone marrow and peripheral blood, recovery of blood counts, and resolution of extra medullary disease) may indicate efficacy.
- Immunoglobulin levels: After treatment.
- Signs and symptoms of cytokine release syndrome: 2 to 3 times during the first week after the infusion at a certified healthcare facility for at least 4 weeks after infusion.
- Signs and symptoms of neurologic toxicities: 2 to 3 times during the first week at a certified healthcare facility; majority of cases occurred within 8 weeks of infusion.
- Hypersensitivity reaction: During infusion.
- Signs and symptoms of infection.
- Secondary malignancies: Life-long.
IV Compatibility
There is limited information regarding the compatibility of Tisagenlecleucel and IV administrations.
Overdosage
There is limited information regarding Tisagenlecleucel overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Tisagenlecleucel
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | None |
| PubChem | ? |
| DrugBank | |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Synonyms | CTL019 |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | 16.8 days |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | Intravenous infusion |
Mechanism of Action
- KYMRIAH is a CD19-directed genetically modified autologous T cell immunotherapy which involves reprogramming a patient’s own T cells with a transgene encoding a chimeric antigen receptor (CAR) to identify and eliminate CD19-expressing malignant and normal cells. The CAR is comprised of a murine single-chain antibody fragment which recognizes CD19 and is fused to intracellular signaling domains from 4-1BB (CD137) and CD3 zeta. The CD3 zeta component is critical for initiating T-cell activation and antitumor activity, while 4-1BB enhances the expansion and persistence of KYMRIAH. Upon binding to CD19-expressing cells, the CAR transmits a signal to promote T-cell expansion, activation, target cell elimination, and persistence of the KYMRIAH cells.
Structure
There is limited information regarding Tisagenlecleucel Structure in the drug label.
Pharmacodynamics
There is limited information regarding Tisagenlecleucel Pharmacodynamics in the drug label.
Pharmacokinetics
- Following infusion, KYMRIAH exhibited an initial rapid expansion followed by a bi-exponential decline in both pediatric and young adult relapsed/refractory B-cell acute lymphoblastic leukemia (ALL) patients, and adult relapsed/refractory diffuse large B-cell lymphoma patients.
- A summary of pharmacokinetic parameters of KYMRIAH is provided in Table 7 below.

Description of Pharmacokinetics in Pediatric and Young Adult r/r B-cell ALL (up to 25 years of age)
- The Cmax and AUC0-28d were approximately 2-fold higher in CR/CRi patients compared with non-responding (NR) patients.
- KYMRIAH was present in the blood as well as bone marrow and was measurable beyond 2 years. Blood to bone marrow partitioning suggested that KYMRIAH distribution in bone marrow was 44% of that present in blood at Day 28 while at Months 3 and 6 KYMRIAH distributed at 67% and 69%, respectively, indicating high distribution to bone marrow.
- Children < 10 years and between 10-18 years of age had 1.5- to 2-fold higher Cmax and AUC0-28d than adults. Due to small sample size and high variability, it is difficult to assess the impact of age on the pharmacokinetics of KYMRIAH.
Description of Pharmacokinetics in Adult r/r DLBCL
- The Cmax and AUC0-28d were similar between responding and non-responding (NR) patients.
- KYMRIAH was present in adult r/r DLBCL patients up to 18 months in peripheral blood and up to 9 months in the bone marrow for patients having a complete response. The median time of maximal expansion of transgene levels (Tmax) in peripheral blood occurred at 9-10 days in both responding and non-responding patients.
Tocilizumab and Corticosteroid use
- Some patients required tocilizumab and corticosteroids for the management of CRS. KYMRIAH continues to expand and persist following tocilizumab administration. Patients who have higher expansion tended to have higher CRS Grades [see Warnings and Precautions (5.1)].
- Pediatric and young adult r/r B-cell ALL patients (n = 18) treated with tocilizumab had 265% and 183% higher KYMRIAH AUC0-28d and Cmax, respectively, as compared to patients (n = 44) who did not receive tocilizumab. In addition, patients who received corticosteroids had 89% higher AUC0-28d compared with patients who did not receive corticosteroids.
- Adult /r/r DLBCL patients treated with tocilizumab (N = 15) had 199% (n = 11) and 257% (n = 13) higher KYMRIAH AUC0-28d and Cmax, respectively, as compared to patients (N = 90) who did not receive tocilizumab. In addition, patients who received corticosteroids (N = 11) had 122% and 161% higher AUC0-28d and Cmax, respectively, as compared with patients who did not receive corticosteroids (N = 94). Hepatic and renal impairment studies of KYMRIAH were not conducted.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Genotoxicity assays and carcinogenicity assessment in rodent models were not performed for KYMRIAH. In vitro expansion studies with transduced T cells (KYMRIAH) from healthy donors and patients showed no evidence for transformation and/or immortalization of T cells. In vivo studies in immunocompromised mice did not show signs of abnormal cell growth or signs of clonal cell expansion for up to 7 months after cell injection. A genomic insertion site analysis was performed on KYMRIAH products from 14 individual donors (12 patients and 2 healthy volunteers). There was no evidence for preferential integration near genes of concern, or preferential outgrowth of cells harboring integration sites of concern.
- No studies on the effects of KYMRIAH on fertility have been conducted.
Clinical Studies
Relapsed or Refractory (r/r) B-cell Acute Lymphoblastic Leukemia (ALL)
- The efficacy of KYMRIAH in pediatric and young adults with r/r B-cell precursor ALL was evaluated in an open-label, multicenter single-arm trial (ELIANA, NCT02228096). In total, 107 patients were screened, 88 were enrolled, 68 were treated, and 63 were evaluable for efficacy. Nine percent of the enrolled patients did not receive the product due to manufacturing failure. The 63 evaluable patients included 35 males and 28 females of median age 12 years (range: 3-23 years). Seventy-three percent of patients were White, 10% were Asian, and 17% were of other races. Six (10%) had primary refractory disease, 30 (48%) had one prior stem cell transplantation, 5 patients (8%) had two stem cell transplantations. Treatment consisted of lymphodepleting chemotherapy (fludarabine 30 mg/m2 daily for 4 days and cyclophosphamide 500 mg/m2 daily for 2 days) followed by a single dose of KYMRIAH. Of the 22 patients who had a WBC count < 1000/µL, 20 received lymphodepleting chemotherapy prior to KYMRIAH while 2 received KYMRIAH infusion without lymphodepleting chemotherapy. Fifty-three patients received bridging chemotherapy between time of enrollment and lymphodepleting chemotherapy.
- The efficacy of KYMRIAH was established on the basis of complete remission (CR) within 3 months after infusion, the duration of CR, and proportion of patients with CR and minimal residual disease (MRD) < 0.01% by flow cytometry (MRD-negative) (Table 8). Among the 63 infused patients, 52 (83%) achieved CR/CRi, all of which were MRD-negative. With a median follow-up of 4.8 months from response, the median duration of CR/CRi was not reached (range: 1.2 to 14.1+ months). Median time to onset of CR/CRi was 29 days with onset of CR/CRi between 26 and 31 days for 50/52 (96%) responders. The stem cell transplantation rate among those who achieved CR/CRi was 12% (6/52). Table 8 shows the efficacy results from this study.

Adult Relapsed or Refractory (r/r) Diffuse Large B-cell Lymphoma (DLBCL)
- The efficacy and safety of KYMRIAH was evaluated in an open-label, multicenter, single-arm trial (JULIET; NCT02445248). Eligible patients were ≥ 18 years of age with relapsed or refractory DLBCL, who received ≥ 2 lines of chemotherapy, including rituximab and anthracycline, or relapsed following autologous hematopoietic stem cell transplantation (HSCT). The study excluded patients with active central nervous system malignancy, prior allogenic HSCT, an ECOG performance status ≥ 2, a creatinine clearance < 60, alanine aminotransferase > 5 times normal, cardiac ejection fraction < 45%, or absolute lymphocyte concentration less than 300/µL.
- Following 2 to 11 days after completion of lymphodepleting (LD) chemotherapy consisting of either fludarabine (25 mg/m2 i.v. daily for 3 days) and cyclophosphamide (250 mg/m2 i.v. daily for 3 days starting with the first dose of fludarabine) or bendamustine (90 mg/m2 i.v. daily for 2 days), KYMRIAH was administered as a single intravenous infusion. Bridging chemotherapy between leukapheresis and LD chemotherapy was permitted to control disease burden. LD chemotherapy could be omitted if the white blood cell count was < 1000 cells/µL. The major efficacy outcome measures were objective response rate per Lugano criteria [2014] as assessed by an independent review committee and duration of response.
- Of the 160 patients enrolled, 106 patients received tisagenlecleucel, including 92 patients who received product manufactured in the U.S. and were followed for at least 3 months or discontinued earlier. Eleven out of 160 patients enrolled did not receive tisagenlecleucel due to manufacturing failure. Thirty-eight other patients did not receive tisagenlecleucel, primarily due to death (n = 16), physician decision (n = 16), and adverse events (n = 3).
- Of the 92 patients receiving KYMRIAH, 90% received physician’s choice of bridging chemotherapy in the interval between start of screening and KYMRIAH infusion, among whom the median number of bridging chemotherapy regimens was 1 (range: 1 to 5) with 83% of patients receiving ≤ 2 regimens. A retrospectively identified sub-group of 68 patients was evaluable for the major efficacy outcome measures. Patients included in this sub-group had either had no bridging chemotherapy, or had imaging that showed measurable disease after completion of bridging chemotherapy, prior to KYMRIAH infusion. Of the 24 patients not included, 8 had no evidence of disease at baseline prior to KYMRIAH infusion, 15 did not have baseline imaging following bridging chemotherapy, and 1 was excluded because of initial misclassification of a neuroendocrine tumor as DLBCL.
- Among the efficacy evaluable population of 68 patients, the baseline characteristics were: median age 56 years (range: 22 to 74 years); 71% male; 90% White, 4% Asian, and 3% Black or African American; 78% had primary DLBCL not otherwise specified (NOS) and 22% had DLBCL following transformation from follicular lymphoma, of whom 17% were identified as high grade; and 44% had undergone prior autologous HSCT. The median number of prior therapies was 3 (range: 1 to 6), 56% had refractory disease and 44% relapsed after their last therapy. Ninety percent of patients received lymphodepleting chemotherapy (66% of patients received fludarabine and 24% received bendamustine) and 10% did not receive any LD chemotherapy. The median time from leukapheresis and cryopreservation to KYMRIAH infusion was 113 days (range: 47 to 196 days). The median dose was 3.5 × 108 CAR-positive viable T cells (range: 1.0 to 5.2 × 108 cells). Seventy-three percent of patients received KYMRIAH in the inpatient setting.
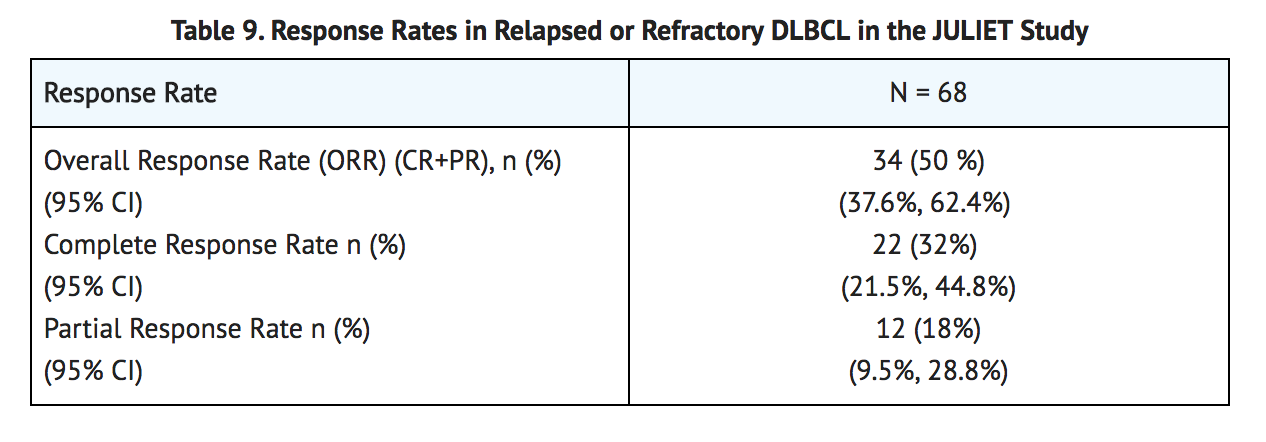
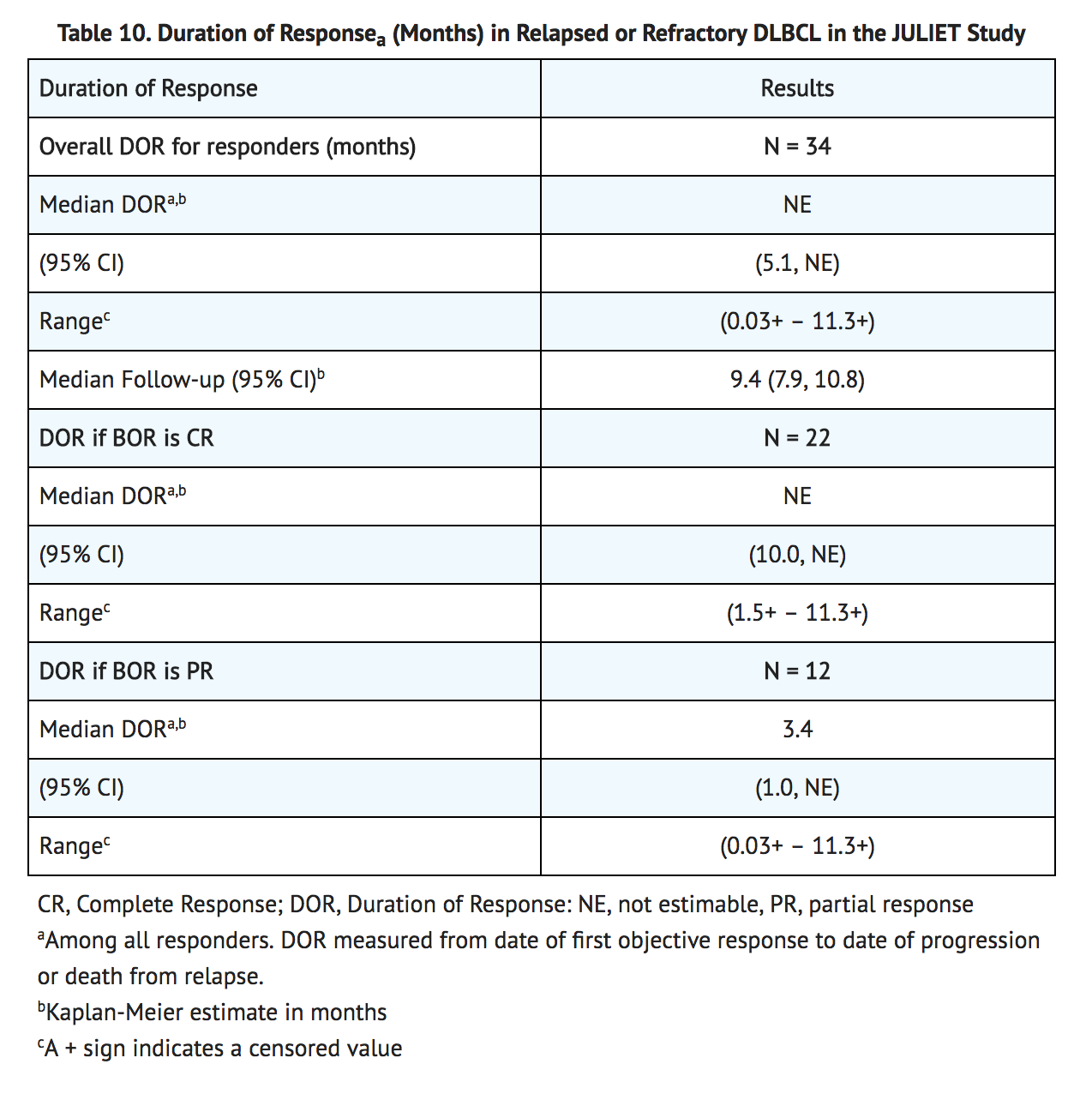
- Efficacy was established on the basis of complete response (CR) rate and duration of response (DOR), as determined by an independent review committee (Table 9 and Table 10). The median time to first response to KYMRIAH (CR or PR) was 0.9 months (range: 0.7 to 3.3 months). The median duration of response was not reached. Response durations were longer in patients who achieved CR, as compared to patients with a best response of partial response (PR) (Table 12). Of the 22 patients who experienced a CR, 9 achieved this status by 1 month, 12 more by month 3, and the last by month 6 after KYMRIAH infusion.
How Supplied
- KYMRIAH is supplied as a frozen suspension of genetically modified autologous T cells in an infusion bag(s) labeled for the specific recipient. KYMRIAH is shipped directly to the cell lab associated with the infusion center in a liquid nitrogen Dewar. Product and patient-specific labels are located inside the Dewar.
- Ped ALL: NDC 0078-0846-19
- DLBCL: NDC 0078-0958-19
Storage
- Confirm patient identity upon receipt.
- Store infusion bag(s) in the vapor phase of liquid nitrogen (less than or equal to minus 120°C) in a temperature-monitored system.
- Use closed, break-proof, leak-proof containers when transporting infusion bags within the facility.
- Thaw KYMRIAH prior to infusion.
Images
Drug Images
{{#ask: Page Name::Tisagenlecleucel |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Tisagenlecleucel |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Ensure that patients understand the risk of manufacturing failure. This has been reported in up to 9% of manufacturing attempts. In case of a manufacturing failure, a second manufacturing of KYMRIAH may be attempted. In addition, while the patient awaits the product, additional chemotherapy (not the lymphodepletion) may be necessary and may increase the risk of adverse events during the pre-infusion period.
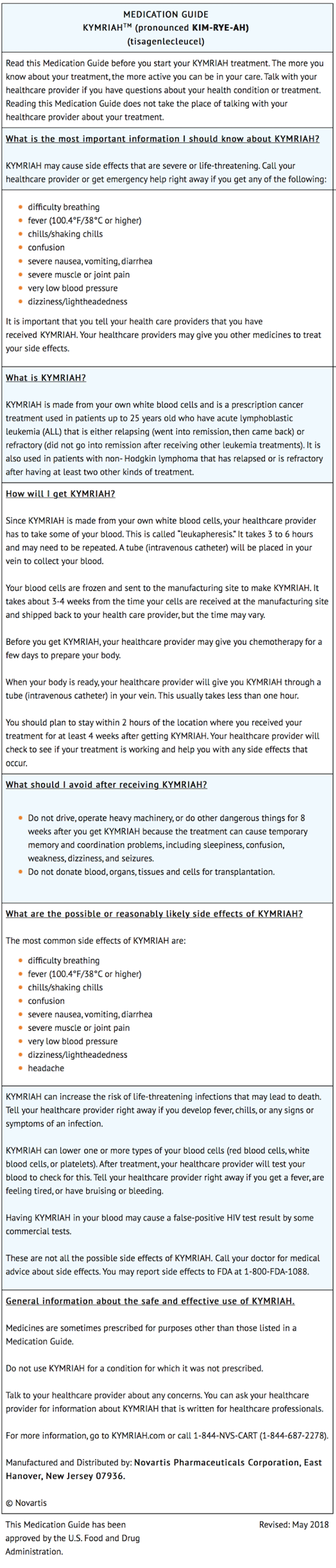
- Prior to infusion, advise patients of the following risks:
- Cytokine Release Syndrome (CRS) -- Report signs and symptoms of CRS (high fever, difficulty breathing, chills/shaking chills, severe nausea, severe vomiting, severe diarrhea, severe muscle pain, severe joint pain, very low blood pressure, or dizziness/lightheadedness) to their healthcare professional.
- Neurological Toxicities -- Report altered or decreased consciousness, delirium, confusion, agitation, seizures, difficulty speaking and understanding, or loss of balance to their healthcare professional.
- Serious Infections -- KYMRIAH may cause serious infections. Advise patients that they will be screened for HBV, HCV, and HIV before collection of cells.
- Hypogammaglobulinemia -- Patients may need to receive immunoglobulin replacement for an indefinite amount of time following treatment with KYMRIAH. Patients should tell their physician about their treatment with KYMRIAH before receiving a live virus vaccine.
- Driving and Engaging in Hazardous Occupations -- Patients should refrain from driving and engaging in hazardous occupations or activities, such as operating heavy or potentially dangerous machinery, for at least 8 weeks after treatment.
- Prolonged Cytopenia -- Patient may exhibit signs or symptoms associated with bone marrow suppression (i.e., neutropenia, thrombocytopenia and anemia) for several weeks following lymphodepleting chemotherapy and KYMRIAH.
- Patients should be instructed to contact Novartis Pharmaceuticals Corporation at 1-844-4KYMRIAH if they get secondary malignancies.
Precautions with Alcohol
Alcohol-Tisagenlecleucel interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Kymriah
Look-Alike Drug Names
There is limited information regarding Tisagenlecleucel Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.