Tildrakizumab
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sonya Gelfand
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Warning Title
See full prescribing information for complete Boxed Warning.
Condition Name: (Content)
|
Overview
Tildrakizumab is a Acetylcholine release inhibitor, Adrenergic receptor agonist that is FDA approved for the (type of indication of drug) of a list of indications, separated by commas.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include a list of adverse reactions, separated by commas..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
CONTRAINDICATIONS
Warnings
|
Warning Title
See full prescribing information for complete Boxed Warning.
Condition Name: (Content)
|
Conidition 1
(Description)
Conidition 2
(Description)
Conidition 3
(Description)
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Condition 2
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
(Description)
Drug Interactions
- Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
(Description)
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Tildrakizumab in women who are pregnant.
Labor and Delivery
(Description)
Nursing Mothers
(Description)g
Pediatric Use
(Description)
Geriatic Use
(Description)
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
- An improvement in signs and symptoms of moderate-to-severe plaque psoriasis is indicative of efficacy.
- Tuberculosis (TB) screening: Prior to initiation.
- Signs and symptoms of active TB: During and after treatment.
IV Compatibility
There is limited information regarding the compatibility of Tildrakizumab and IV administrations.
Overdosage
- In the event of overdosage, monitor the patient for any signs or symptoms of adverse reactions and administer appropriate symptomatic treatment immediately.
Pharmacology
Mechanism of Action
- Tildrakizumab is a humanized IgG1/k monoclonal antibody that selectively binds to the p19 subunit of IL-23 and inhibits its interaction with the IL-23 receptor. IL-23 is a naturally occurring cytokine that is involved in inflammatory and immune responses. Tildrakizumab inhibits the release of proinflammatory cytokines and chemokines.
Structure
(Description with picture)
Pharmacodynamics
- No formal pharmacodynamics studies have been conducted with ILUMYA.
Pharmacokinetics
- Tildrakizumab pharmacokinetics increases proportionally over a dose range from 50 mg to 200 mg (0.5 to 2 times the approved recommended dosage) following subcutaneous administration in subjects with plaque psoriasis. Steady-state concentrations were achieved by Week 16 following subcutaneous administration of tildrakizumab at Weeks 0, 4, and every 12 weeks thereafter. At the 100 mg dose at Week 16, the mean (± SD) steady-state trough concentrations ranged from 1.22 ± 0.94 mcg/mL to 1.47 ± 1.12 mcg/mL. The geometric mean (CV%) steady-state Cmax was 8.1 mcg/mL (34%).
Absorption
- The absolute bioavailability of tildrakizumab was estimated to be 73-80% following subcutaneous injection. The peak concentration (Cmax) was reached by approximately 6 days.
Distribution
- The geometric mean (CV%) volume of distribution is 10.8 L (24%).
Elimination
- The geometric mean (CV%) systemic clearance was 0.32 L/day (38%) and the half-life was approximately 23 days (23%).
Metabolism
- The metabolic pathway of tildrakizumab has not been characterized. As a humanized IgG1/k monoclonal antibody, tildrakizumab is expected to be degraded into small peptides and amino acids via catabolic pathways in a manner similar to endogenous IgG.
Specific Populations
- No clinically significant differences in the pharmacokinetics of tildrakizumab were observed based on age (≥18 years). No specific studies have been conducted to determine the effect of renal or hepatic impairment on the pharmacokinetics of tildrakizumab.
Body Weight
- Tildrakizumab concentrations were lower in subjects with higher body weight.
Drug Interaction Studies
Cytochrome P450 Substrates
- The AUCinf of dextromethorphan (CYP2D6 substrate) increased by 20% when used concomitantly with tildrakizumab 200 mg (two times the approved recommended dose) administered subcutaneously at Weeks 0 and 4 in subjects with plaque psoriasis. No clinically significant changes in AUCinf of caffeine (CYP1A2 substrate), warfarin (CYP2C9 substrate), omeprazole (CYP2C19 substrate), and midazolam (CYP3A4 substrate) were observed.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Animal studies have not been conducted to evaluate the carcinogenic or mutagenic potential of ILUMYA.
- No effects on fertility parameters were observed in male or female cynomolgus monkeys that were administered tildrakizumab at subcutaneous or intravenous doses up to 140 mg/kg once every two weeks for 3 months (133 or 155 times the MRHD, respectively, based on AUC comparison). The monkeys were not mated to evaluate fertility.
Clinical Studies
- In two multicenter, randomized, double-blind, placebo-controlled trials (Trial 2 [NCT01722331] and Trial 3 [NCT01729754]), 926 subjects were treated with ILUMYA 100 mg (N=616) or placebo (N=310). Subjects had a Physician Global Assessment (PGA) score of ≥3 (moderate) on a 5-point scale of overall disease severity, Psoriasis Area and Severity Index (PASI) score ≥12, and a minimum body surface area (BSA) involvement of 10%. Subjects with guttate, erythrodermic, or pustular psoriasis were excluded.
- In both trials, subjects were randomized to either placebo or ILUMYA (100 mg at Week 0, Week 4 and every twelve weeks thereafter [Q12W]) up to 64 weeks.
- Trials 2 and 3 assessed the changes from baseline to Week 12 in the two co-primary endpoints:
- PASI 75, the proportion of subjects who achieved at least a 75% reduction in the PASI composite score.
- PGA of 0 (“cleared”) or 1 (“minimal”), the proportion of subjects with a PGA of 0 or 1 and at least a 2-point improvement.
- Other evaluated outcomes in Trials 2 and 3 included the proportion of subjects who achieved a reduction from baseline in PASI score of at least 90% (PASI 90) and a reduction of 100% in PASI score (PASI 100) at Week 12 and maintenance of efficacy up to Week 64.
- In both trials, subjects in the ILUMYA 100 mg and placebo treatment groups were predominantly men (69%) and White (80%), with a mean age of 46 years. At baseline, these subjects had a median affected BSA of 27%, a median PASI score of 17.8, and approximately 33% had a PGA score of 4 (“marked”) or 5 (“severe”). Approximately 34% had received prior phototherapy, 39% had received prior conventional systemic therapy, and 18% had received prior biologic therapy for the treatment of psoriasis. Approximately 16% of subjects had a history of psoriatic arthritis.
Clinical Response at Week 12
- The results of Trials 2 and 3 are presented in Table 2.
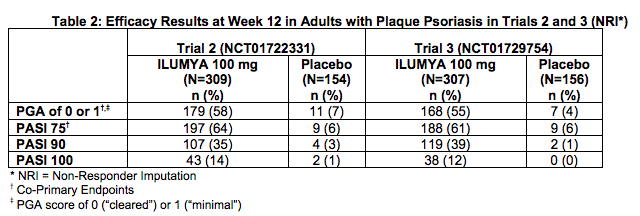
- Examination of age, gender, race, and previous treatment with a biologic did not identify differences in response to ILUMYA among these subgroups at Week 12.
Maintenance of Response and Durability of Response
- In Trial 2, subjects originally randomized to ILUMYA and who were responders at Week 28 (i.e., PASI 75) were re-randomized to an additional 36 weeks of either maintaining the same dose of ILUMYA Q12W (every twelve weeks) or placebo.
- At Week 28, 229 (74%) subjects treated with ILUMYA 100 mg were PASI 75 responders. At Week 64, 84% of subjects who continued on ILUMYA 100 mg Q12W maintained PASI 75 compared to 22% of subjects who were re-randomized to placebo. In addition, for subjects who were re-randomized and also had a PGA score of 0 or 1 at Week 28, 69% of subjects who continued on ILUMYA 100 mg Q12W maintained this response (PGA 0 or 1) at Week 64 compared to 14% of subjects who were rerandomized to placebo.
- For PASI 75 responders at Week 28 who were re-randomized to treatment withdrawal (i.e., placebo), the median time to loss of PASI 75 was approximately 20 weeks.
- In addition, for subjects who were re-randomized to placebo and also had a PGA score of 0 or 1 at Week 28, the median time to loss of PGA score of 0 or 1 was approximately 16 weeks.
How Supplied
- ILUMYA (tildrakizumab-asmn) Injection is a sterile, preservative-free, clear to slightly opalescent, colorless to slightly yellow solution. ILUMYA is supplied as one single-dose prefilled syringe per carton that delivers 1 mL of a 100 mg/mL solution.
- NDC 0006-4241-00
- Each prefilled syringe is equipped with a passive needle guard and a needle cover.
Storage
- Store refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light until the time of use. Do not freeze. Do not shake. ILUMYA can be kept at room temperature at 25°C (77°F) for up to 30 days in the original carton to protect from light. Once stored at room temperature, do not place back in the refrigerator. If not used within 30 days, discard ILUMYA. Do not store ILUMYA above 25°C (77°F).
Images
Drug Images
{{#ask: Page Name::Tildrakizumab |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Tildrakizumab |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patient and/or caregiver to read the FDA-approved patient labeling.
- Instruct patients and/or caregivers to read the Medication Guide before starting ILUMYA therapy and to reread the Medication Guide each time the prescription is renewed. Advise patients of the potential benefits and risks of ILUMYA.
Hypersensitivity
- Advise patients to seek immediate medical attention if they experience any symptoms of serious hypersensitivity reactions.
Infections
- Instruct patients of the importance of communicating any history of infections to the doctor and contacting their doctor if they develop any symptoms of infection
Precautions with Alcohol
Alcohol-Tildrakizumab interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Ilumya
Look-Alike Drug Names
There is limited information regarding Tildrakizumab Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.