Tamoxifen
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Deepika Beereddy, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
For Women With Ductal Carcinoma in Situ (DCIS) and Women at High Risk for Breast Cancer:
Serious and life-threatening events associated with tamoxifen in the risk reduction setting (women at high risk for cancer and women with DCIS) include uterine malignancies, stroke and pulmonary embolism. Incidence rates for these events were estimated from the NSABP P-1 trial (see CLINICAL PHARMACOLOGY, Clinical Studies, Reduction in Breast Cancer Incidence in High Risk Women). Uterine malignancies consist of both endometrial adenocarcinoma (incidence rate per 1,000 women-years of 2.20 for tamoxifen vs. 0.71 for placebo) and uterine sarcoma (incidence rate per 1,000 women-years of 0.17 for tamoxifen vs. 0.4 for placebo)*. For stroke, the incidence rate per 1,000 women-years was 1.43 for tamoxifen vs. 1.00 for placebo**. For pulmonary embolism, the incidence rate per 1,000 women-years was 0.75 for tamoxifen versus 0.25 for placebo**. Some of the strokes, pulmonary emboli, and uterine malignancies were fatal. Health care providers should discuss the potential benefits versus the potential risks of these serious events with women at high risk of breast cancer and women with DCIS considering tamoxifen to reduce their risk of developing breast cancer. The benefits of tamoxifen outweigh its risks in women already diagnosed with breast cancer.
|
Overview
Tamoxifen is an antineoplastic agent, bone density conservation agent that is FDA approved for the treatment of {{{indication}}}. There is a Black Box Warning for this drug as shown here. Common adverse reactions include .
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Breast cancer, Adjuvant therapy
- Tamoxifen citrate tablets are indicated for the treatment of node-positive breast cancer in women following total mastectomy or segmental mastectomy, axillary dissection, and breast irradiation. In some tamoxifen adjuvant studies, most of the benefit to date has been in the subgroup with four or more positive axillary nodes.
Tamoxifen citrate tablets are indicated for the treatment of axillary node-negative breast cancer in women following total mastectomy or segmental mastectomy, axillary dissection, and breast irradiation.
The estrogen and progesterone receptor values may help to predict whether adjuvant tamoxifen therapy is likely to be beneficial.
Tamoxifen reduces the occurrence of contralateral breast cancer in patients receiving adjuvant tamoxifen therapy for breast cancer.
- Dosing Information
- For patients with breast cancer, the recommended daily dose is 20 to 40 mg. Dosages greater than 20 mg per day should be given in divided doses (morning and evening).
In three single agent adjuvant studies in women, one 10 mg tamoxifen citrate tablet was administered two (ECOG and NATO) or three (Toronto) times a day for two years. In the NSABP B-14 adjuvant study in women with node-negative breast cancer, one 10 mg tamoxifen citrate tablet was given twice a day for at least 5 years. Results of the B-14 study suggest that continuation of therapy beyond five years does not provide additional benefit (see CLINICAL PHARMACOLOGY). In the EBCTCG 1995 overview, the reduction in recurrence and mortality was greater in those studies that used tamoxifen for about 5 years than in those that used tamoxifen for a shorter period of therapy. There was no indication that doses greater than 20 mg per day were more effective. Current data from clinical trials support 5 years of adjuvant tamoxifen therapy for patients with breast cancer.
Breast cancer, High-risk; Prophylaxis
Tamoxifen citrate tablets are indicated to reduce the incidence of breast cancer in women at high risk for breast cancer. This effect was shown in a study of 5 years planned duration with a median follow-up of 4.2 years. Twenty-five percent of the participants received drug for 5 years. The longer-term effects are not known. In this study, there was no impact of tamoxifen on overall or breast cancer-related mortality (see BOXED WARNING at the beginning of the label).
Tamoxifen citrate tablets are indicated only for high-risk women. “High risk” is defined as women at least 35 years of age with a 5 year predicted risk of breast cancer ≥ 1.67%, as calculated by the Gail Model.
Examples of combinations of factors predicting a 5 year risk ≥ 1.67% are:
Age 35 or older and any of the following combination of factors:
One first degree relative with a history of breast cancer, 2 or more benign biopsies, and a history of a breast biopsy showing atypical hyperplasia; or At least 2 first degree relatives with a history of breast cancer, and a personal history of at least 1 breast biopsy; or LCIS
Age 40 or older and any of the following combination of factors:
One first degree relative with a history of breast cancer, 2 or more benign biopsies, age at first live birth 25 or older, and age at menarche 11 or younger; or At least 2 first degree relatives with a history of breast cancer, and age at first live birth 19 or younger; or One first degree relative with a history of breast cancer, and a personal history of a breast biopsy showing atypical hyperplasia.
Age 45 or older and any of the following combination of factors:
At least 2 first degree relatives with a history of breast cancer and age at first live birth 24 or younger; or One first degree relative with a history of breast cancer with a personal history of a benign breast biopsy, age at menarche 11 or less and age at first live birth 20 or more.
Age 50 or older and any of the following combination of factors:
At least 2 first degree relatives with a history of breast cancer; or History of 1 breast biopsy showing atypical hyperplasia, and age at first live birth 30 or older and age at menarche 11 or less; or History of at least 2 breast biopsies with a history of atypical hyperplasia, and age at first live birth 30 or more.
Age 55 or older and any of the following combination of factors:
One first degree relative with a history of breast cancer with a personal history of a benign breast biopsy, and age at menarche 11 or less; or History of at least 2 breast biopsies with a history of atypical hyperplasia, and age at first live birth 20 or older.
Age 60 or older and:
Five-year predicted risk of breast cancer ≥ 1.67%, as calculated by the Gail Model.
For women whose risk factors are not described in the above examples, the Gail Model is necessary to estimate absolute breast cancer risk. Health Care Professionals can obtain a Gail Model Risk Assessment Tool by dialing 1-888-838-2872.
There are insufficient data available regarding the effect of tamoxifen on breast cancer incidence in women with inherited mutations (BRCA1, BRCA2) to be able to make specific recommendations on the effectiveness of tamoxifen in these patients.
After an assessment of the risk of developing breast cancer, the decision regarding therapy with tamoxifen for the reduction in breast cancer incidence should be based upon an individual assessment of the benefits and risks of tamoxifen therapy. In the NSABP P-1 trial, tamoxifen treatment lowered the risk of developing breast cancer during the follow-up period of the trial, but did not eliminate breast cancer risk (see Table 3 in CLINICAL PHARMACOLOGY).
- Dosing Information
- The recommended dose is tamoxifen 20 mg daily for 5 years. There are no data to support the use of tamoxifen other than for 5 years (see CLINICAL PHARMACOLOGY, Clinical Studies, Reduction in Breast Cancer Incidence in High Risk Women).
Intraductal carcinoma in situ of breast, Following breast surgery and radiation, to reduce risk of invasive disease
In women with DCIS, following breast surgery and radiation, tamoxifen citrate tablets are indicated to reduce the risk of invasive breast cancer (see BOXED WARNING at the beginning of the label). The decision regarding therapy with tamoxifen for the reduction in breast cancer incidence should be based upon an individual assessment of the benefits and risks of tamoxifen therapy.
Current data from clinical trials support 5 years of adjuvant tamoxifen therapy for patients with breast cancer.
- Dosing Information
- The recommended dose is tamoxifen 20 mg daily for 5 years.
Malignant neoplasm of male breast, Metastatic
- Dosing Information
- Dosage
Metastatic breast cancer
- Tamoxifen citrate tablets are effective in the treatment of metastatic breast cancer in women and men. In premenopausal women with metastatic breast cancer, tamoxifen is an alternative to oophorectomy or ovarian irradiation. Available evidence indicates that patients whose tumors are estrogen receptor positive are more likely to benefit from tamoxifen therapy.
- Dosing Information
- Dosage
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Tamoxifen in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Tamoxifen in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Tamoxifen in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Tamoxifen in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Tamoxifen in pediatric patients.
Contraindications
- Condition1
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
For Women With Ductal Carcinoma in Situ (DCIS) and Women at High Risk for Breast Cancer:
Serious and life-threatening events associated with tamoxifen in the risk reduction setting (women at high risk for cancer and women with DCIS) include uterine malignancies, stroke and pulmonary embolism. Incidence rates for these events were estimated from the NSABP P-1 trial (see CLINICAL PHARMACOLOGY, Clinical Studies, Reduction in Breast Cancer Incidence in High Risk Women). Uterine malignancies consist of both endometrial adenocarcinoma (incidence rate per 1,000 women-years of 2.20 for tamoxifen vs. 0.71 for placebo) and uterine sarcoma (incidence rate per 1,000 women-years of 0.17 for tamoxifen vs. 0.4 for placebo)*. For stroke, the incidence rate per 1,000 women-years was 1.43 for tamoxifen vs. 1.00 for placebo**. For pulmonary embolism, the incidence rate per 1,000 women-years was 0.75 for tamoxifen versus 0.25 for placebo**. Some of the strokes, pulmonary emboli, and uterine malignancies were fatal. Health care providers should discuss the potential benefits versus the potential risks of these serious events with women at high risk of breast cancer and women with DCIS considering tamoxifen to reduce their risk of developing breast cancer. The benefits of tamoxifen outweigh its risks in women already diagnosed with breast cancer.
|
- Description
Precautions
- Description
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Tamoxifen in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Tamoxifen in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Tamoxifen in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Tamoxifen during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Tamoxifen with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Tamoxifen with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Tamoxifen with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Tamoxifen with respect to specific gender populations.
Race
There is no FDA guidance on the use of Tamoxifen with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Tamoxifen in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Tamoxifen in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Tamoxifen in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Tamoxifen in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Tamoxifen in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Tamoxifen in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Tamoxifen in the drug label.
Pharmacology

Mechanism of Action
Tamoxifen citrate is a nonsteroidal agent that has demonstrated potent antiestrogenic properties in animal test systems. The antiestrogenic effects may be related to its ability to compete with estrogen for binding sites in target tissues such as breast. Tamoxifen inhibits the induction of rat mammary carcinoma induced by dimethylbenzanthracene (DMBA) and causes the regression of already established DMBA-induced tumors. In this rat model, tamoxifen appears to exert its antitumor effects by binding the estrogen receptors.
In cytosols derived from human breast adenocarcinomas, tamoxifen competes with estradiol for estrogen receptor protein.
Structure
Tamoxifen citrate tablets USP, a nonsteroidal antiestrogen, are for oral administration. Each tablet contains 10 mg or 20 mg tamoxifen (equivalent to 15.2 mg or 30.4 mg, respectively, of tamoxifen citrate).
Each tablet contains the following inactive ingredients: croscarmellose sodium, hypromellose, lactose (monohydrate), magnesium stearate, polyethylene glycol 400, povidone, corn starch, and titanium dioxide.
Chemically, tamoxifen is the trans-isomer of a triphenylethylene derivative. The chemical name is (Z)2-(4-(1,2-diphenyl-1-butenyl)phenoxy)- N,N-dimethylethanamine 2-hydroxy-1,2,3- propanetricarboxylate (1:1). The structural formula, empirical formula, and molecular weight are as follows:

C32H37NO8 M.W. 563.62
Tamoxifen citrate has a pKa’ of 8.85, the equilibrium solubility in water at 37°C is 0.5 mg/mL and in 0.02 N HCl at 37°C, it is 0.2 mg/mL.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Tamoxifen in the drug label.
Pharmacokinetics
Absorption and Distribution
Following a single oral dose of 20 mg tamoxifen, an average peak plasma concentration of 40 ng/mL (range 35 to 45 ng/mL) occurred approximately 5 hours after dosing. The decline in plasma concentrations of tamoxifen is biphasic with a terminal elimination half-life of about 5 to 7 days. The average peak plasma concentration of N-desmethyl tamoxifen is 15 ng/mL (range 10 to 20 ng/mL). Chronic administration of 10 mg tamoxifen given twice daily for 3 months to patients results in average steady-state plasma concentrations of 120 ng/mL (range 67 to 183 ng/mL) for tamoxifen and 336 ng/mL (range 148 to 654 ng/mL) for N-desmethyl tamoxifen. The average steady-state plasma concentrations of tamoxifen and N-desmethyl tamoxifen after administration of 20 mg tamoxifen once daily for 3 months are 122 ng/mL (range 71 to 183 ng/mL) and 353 ng/mL (range 152 to 706 ng/mL), respectively. After initiation of therapy, steady-state concentrations for tamoxifen are achieved in about 4 weeks and steady-state concentrations for N-desmethyl tamoxifen are achieved in about 8 weeks, suggesting a half-life of approximately 14 days for this metabolite. In a steady-state, crossover study of 10 mg tamoxifen citrate tablets given twice a day vs. a 20 mg tamoxifen citrate tablet given once daily, the 20 mg tamoxifen citrate tablet was bioequivalent to the 10 mg tamoxifen citrate tablets.
Metabolism
Tamoxifen is extensively metabolized after oral administration. N-desmethyl tamoxifen is the major metabolite found in patients’ plasma. The biological activity of N-desmethyl tamoxifen appears to be similar to that of tamoxifen. 4-Hydroxytamoxifen and a side chain primary alcohol derivative of tamoxifen have been identified as minor metabolites in plasma. Tamoxifen is a substrate of cytochrome P-450 3A, 2C9 and 2D6, and an inhibitor of P-glycoprotein.
Excretion
Studies in women receiving 20 mg of 14C tamoxifen have shown that approximately 65% of the administered dose was excreted from the body over a period of 2 weeks with fecal excretion as the primary route of elimination. The drug is excreted mainly as polar conjugates, with unchanged drug and unconjugated metabolites accounting for less than 30% of the total fecal radioactivity.
Special Populations
The effects of age, gender and race on the pharmacokinetics of tamoxifen have not been determined. The effects of reduced liver function on the metabolism and pharmacokinetics of tamoxifen have not been determined.
Pediatric Patients
The pharmacokinetics of tamoxifen and N-desmethyl tamoxifen were characterized using a population pharmacokinetic analysis with sparse samples per patient obtained from 27 female pediatric patients aged 2 to 10 years enrolled in a study designed to evaluate the safety, efficacy, and pharmacokinetics of tamoxifen in treating McCune-Albright syndrome. Rich data from two tamoxifen citrate pharmacokinetic trials in which 59 postmenopausal women with breast cancer completed the studies were included in the analysis to determine the structural pharmacokinetic model for tamoxifen. A one-compartment model provided the best fit to the data.
In pediatric patients, an average steady-state peak plasma concentration (Css, max) and AUC were of 187 ng/mL and 4,110 ng hr/mL, respectively, and Css, max occurred approximately 8 hours after dosing. Clearance (CL/F) as body weight adjusted in female pediatric patients was approximately 2.3 fold higher than in female breast cancer patients. In the youngest cohort of female pediatric patients (2 to 6 year olds), CL/F was 2.6 fold higher; in the oldest cohort (7 to 10.9 year olds) CL/F was approximately 1.9 fold higher. Exposure to N-desmethyl tamoxifen was comparable between the pediatric and adult patients. The safety and efficacy of tamoxifen for girls aged 2 to 10 years with McCune-Albright syndrome and precocious puberty have not been studied beyond one year of treatment. The long-term effects of tamoxifen therapy in girls have not been established. In adults treated with tamoxifen an increase in incidence of uterine malignancies, stroke and pulmonary embolism has been noted (see BOXED WARNING).
Drug-Drug Interactions
In vitro studies showed that erythromycin, cyclosporin, nifedipine and diltiazem competitively inhibited formation of N-desmethyl tamoxifen with apparent K1 of 20, 1, 45 and 30 µM, respectively. The clinical significance of these in vitro studies is unknown.
Tamoxifen reduced the plasma concentration of letrozole by 37% when these drugs were coadministered. Rifampin, a cytochrome P-450 3A4 inducer reduced tamoxifen AUC and Cmax by 86% and 55%, respectively. Aminoglutethimide reduces tamoxifen and N-desmethyl tamoxifen plasma concentrations. Medroxyprogesterone reduces plasma concentrations of N-desmethyl, but not tamoxifen.
In the anastrozole adjuvant trial, coadministration of anastrozole and tamoxifen in breast cancer patients reduced anastrozole plasma concentration by 27% compared to those achieved with anastrozole alone; however, the coadministration did not affect the pharmacokinetics of tamoxifen or N-desmethyltamoxifen (see PRECAUTIONS, Drug Interactions). Tamoxifen should not be coadministered with anastrozole.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Tamoxifen in the drug label.
Clinical Studies
Metastatic Breast Cancer
Premenopausal women (tamoxifen vs. ablation)
Three prospective, randomized studies (Ingle, Pritchard, Buchanan) compared tamoxifen to ovarian ablation (oophorectomy or ovarian irradiation) in premenopausal women with advanced breast cancer. Although the objective response rate, time to treatment failure, and survival were similar with both treatments, the limited patient accrual prevented a demonstration of equivalence. In an overview analysis of survival data from the 3 studies, the hazard ratio for death (tamoxifen/ovarian ablation) was 1.00 with two-sided 95% confidence intervals of 0.73 to 1.37. Elevated serum and plasma estrogens have been observed in premenopausal women receiving tamoxifen, but the data from the randomized studies do not suggest an adverse effect of this increase. A limited number of premenopausal patients with disease progression during tamoxifen therapy responded to subsequent ovarian ablation.
Male breast cancer
Published results from 122 patients (119 evaluable) and case reports in 16 patients (13 evaluable) treated with tamoxifen have shown that tamoxifen is effective for the palliative treatment of male breast cancer. Sixty-six of these 132 evaluable patients responded to tamoxifen which constitutes a 50% objective response rate.
Adjuvant Breast Cancer
Overview
The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) conducted worldwide overviews of systemic adjuvant therapy for early breast cancer in 1985, 1990, and again in 1995. In 1998, 10 year outcome data were reported for 36,689 women in 55 randomized trials of adjuvant tamoxifen using doses of 20 to 40 mg/day for 1 to 5+ years. Twenty-five percent of patients received 1 year or less of trial treatment, 52% received 2 years, and 23% received about 5 years. Forty-eight percent of tumors were estrogen receptor (ER) positive (> 10 fmol/mg), 21% were ER poor (< 10 fmol/l), and 31% were ER unknown. Among 29,441 patients with ER positive or unknown breast cancer, 58% were entered into trials comparing tamoxifen to no adjuvant therapy and 42% were entered into trials comparing tamoxifen in combination with chemotherapy vs. the same chemotherapy alone. Among these patients, 54% had node positive disease and 46% had node negative disease.
Among women with ER positive or unknown breast cancer and positive nodes who received about 5 years of treatment, overall survival at 10 years was 61.4% for tamoxifen vs. 50.5% for control (logrank 2p < 0.00001). The recurrence-free rate at 10 years was 59.7% for tamoxifen vs. 44.5% for control (logrank 2p < 0.00001). Among women with ER positive or unknown breast cancer and negative nodes who received about 5 years of treatment, overall survival at 10 years was 78.9% for tamoxifen vs. 73.3% for control (logrank 2p < 0.00001). The recurrence-free rate at 10 years was 79.2% for tamoxifen vs. 64.3% for control (logrank 2p < 0.00001).
The effect of the scheduled duration of tamoxifen may be described as follows. In women with ER positive or unknown breast cancer receiving 1 year or less, 2 years or about 5 years of tamoxifen, the proportional reductions in mortality were 12%, 17% and 26%, respectively (trend significant at 2p < 0.003). The corresponding reductions in breast cancer recurrence were 21%, 29% and 47% (trend significant at 2p < 0.00001).
Benefit is less clear for women with ER poor breast cancer in whom the proportional reduction in recurrence was 10% (2p = 0.007) for all durations taken together, or 9% (2p = 0.02) if contralateral breast cancers are excluded. The corresponding reduction in mortality was 6% (NS). The effects of about 5 years of tamoxifen on recurrence and mortality were similar regardless of age and concurrent chemotherapy. There was no indication that doses greater than 20 mg per day were more effective.
Anastrozole adjuvant ATAC trial – study of anastrozole compared to tamoxifen for adjuvant treatment of early breast cancer
An anastrozole adjuvant trial was conducted in 9,366 postmenopausal women with operable breast cancer who were randomized to receive adjuvant treatment with either anastrozole 1 mg daily, tamoxifen 20 mg daily, or a combination of these two treatments for 5 years or until recurrence of the disease. At a median follow-up of 33 months, the combination of anastrozole and tamoxifen did not demonstrate any efficacy benefit when compared with tamoxifen therapy alone in all patients as well as in the hormone receptor-positive subpopulation. This treatment arm was discontinued from the trial. Please refer to CLINICAL PHARMACOLOGY, Special Populations and Drug-Drug Interactions; PRECAUTIONS, Laboratory Tests; PRECAUTIONS, Drug Interactions and ADVERSE REACTIONS for safety information from this trial. Please refer to the full prescribing information for anastrozole 1 mg tablets for additional information on this trial.
Patients in the two monotherapy arms of the ATAC trial were treated for a median of 60 months (5 years) and followed for a median of 68 months. Disease-free survival in the intent-to-treat population was statistically significantly improved [Hazard Ratio (HR) = 0.87, 95% CI: 0.78, 0.97, p = 0.0127] in the anastrozole arm compared to the tamoxifen arm.
Node positive – individual studies
Two studies (Hubay and NSABP B-09) demonstrated an improved disease-free survival following radical or modified radical mastectomy in postmenopausal women or women 50 years of age or older with surgically curable breast cancer with positive axillary nodes when tamoxifen was added to adjuvant cytotoxic chemotherapy. In the Hubay study, tamoxifen was added to "low-dose" CMF (cyclophosphamide, methotrexate and fluorouracil). In the NSABP B-09 study, tamoxifen was added to melphalan [L-phenylalanine mustard (P)] and fluorouracil (F).
In the Hubay study, patients with a positive (more than 3 fmol) estrogen receptor were more likely to benefit. In the NSABP B-09 study in women age 50 to 59 years, only women with both estrogen and progesterone receptor levels 10 fmol or greater clearly benefited, while there was a nonstatistically significant trend toward adverse effect in women with both estrogen and progesterone receptor levels less than 10 fmol. In women age 60 to 70 years, there was a trend toward a beneficial effect of tamoxifen without any clear relationship to estrogen or progesterone receptor status.
Three prospective studies (ECOG-1178, Toronto, NATO) using tamoxifen adjuvantly as a single agent demonstrated an improved disease-free survival following total mastectomy and axillary dissection for postmenopausal women with positive axillary nodes compared to placebo/no treatment controls. The NATO study also demonstrated an overall survival benefit.
Node negative – individual studies
NSABP B-14, a prospective, double-blind, randomized study, compared tamoxifen to placebo in women with axillary node-negative, estrogen-receptor positive (≥ 10 fmol/mg cytosol protein) breast cancer (as adjuvant therapy, following total mastectomy and axillary dissection, or segmental resection, axillary dissection, and breast radiation). After five years of treatment, there was a significant improvement in disease-free survival in women receiving tamoxifen. This benefit was apparent both in women under age 50 and in women at or beyond age 50.
One additional randomized study (NATO) demonstrated improved disease-free survival for tamoxifen compared to no adjuvant therapy following total mastectomy and axillary dissection in postmenopausal women with axillary node-negative breast cancer. In this study, the benefits of tamoxifen appeared to be independent of estrogen receptor status.
Duration of therapy
In the EBCTCG 1995 overview, the reduction in recurrence and mortality was greater in those studies that used tamoxifen for about 5 years than in those that used tamoxifen for a shorter period of therapy.
In the NSABP B-14 trial, in which patients were randomized to tamoxifen 20 mg/day for 5 years vs. placebo and were disease-free at the end of this 5 year period were offered rerandomization to an additional 5 years of tamoxifen or placebo. With 4 years of follow-up after this rerandomization, 92% of the women that received 5 years of tamoxifen were alive and disease-free, compared to 86% of the women scheduled to receive 10 years of tamoxifen (p = 0.003). Overall survivals were 96% and 94%, respectively (p = 0.08). Results of the B-14 study suggest that continuation of therapy beyond 5 years does not provide additional benefit.
A Scottish trial of 5 years of tamoxifen vs. indefinite treatment found a disease-free survival of 70% in the five-year group and 61% in the indefinite group, with 6.2 years median follow-up (HR = 1.27, 95% CI: 0.87 to 1.85).
In a large randomized trial conducted by the Swedish Breast Cancer Cooperative Group of adjuvant tamoxifen 40 mg/day for 2 or 5 years, overall survival at 10 years was estimated to be 80% in the patients in the 5 year tamoxifen group, compared with 74% among corresponding patients in the 2 year treatment group (p = 0.03). Disease-free survival at 10 years was 73% in the 5 year group and 67% in the 2 year group (p = 0.009). Compared with 2 years of tamoxifen treatment, 5 years of treatment resulted in a slightly greater reduction in the incidence of contralateral breast cancer at 10 years, but this difference was not statistically significant.
Contralateral breast cancer
The incidence of contralateral breast cancer is reduced in breast cancer patients (premenopausal and postmenopausal) receiving tamoxifen compared to placebo. Data on contralateral breast cancer are available from 32,422 out of 36,689 patients in the 1995 overview analysis of the Early Breast Cancer Trialists Collaborative Group (EBCTCG). In clinical trials with tamoxifen of 1 year or less, 2 years, and about 5 years duration, the proportional reductions in the incidence rate of contralateral breast cancer among women receiving tamoxifen were 13% (NS), 26% (2p = 0.004) and 47% (2p < 0.00001), with a significant trend favoring longer tamoxifen duration (2p = 0.008). The proportional reductions in the incidence of contralateral breast cancer were independent of age and ER status of the primary tumor. Treatment with about 5 years of tamoxifen reduced the annual incidence rate of contralateral breast cancer from 7.6 per 1,000 patients in the control group compared with 3.9 per 1,000 patients in the tamoxifen group.
In a large randomized trial in Sweden (the Stockholm Trial) of adjuvant tamoxifen 40 mg/day for 2 to 5 years, the incidence of second primary breast tumors was reduced 40% (p < 0.008) on tamoxifen compared to control. In the NSABP B-14 trial in which patients were randomized to tamoxifen 20 mg/day for 5 years vs. placebo, the incidence of second primary breast cancers was also significantly reduced (p < 0.01). In NSABP B-14, the annual rate of contralateral breast cancer was 8.0 per 1,000 patients in the placebo group compared with 5.0 per 1,000 patients in the tamoxifen group, at 10 years after first randomization.
Ductal Carcinoma in Situ
NSABP B-24, a double-blind, randomized trial included women with ductal carcinoma in situ (DCIS). This trial compared the addition of tamoxifen or placebo to treatment with lumpectomy and radiation therapy for women with DCIS. The primary objective was to determine whether 5 years of tamoxifen therapy (20 mg/day) would reduce the incidence of invasive breast cancer in the ipsilateral (the same) or contralateral (the opposite) breast.
In this trial 1,804 women were randomized to receive either tamoxifen or placebo for 5 years: 902 women were randomized to tamoxifen citrate 10 mg tablets twice a day and 902 women were randomized to placebo. As of December 31, 1998, follow-up data were available for 1,798 women and the median duration of follow-up was 74 months.
The tamoxifen and placebo groups were well balanced for baseline demographic and prognostic factors. Over 80% of the tumors were less than or equal to 1 cm in their maximum dimension, were not palpable, and were detected by mammography alone. Over 60% of the study population was postmenopausal. In 16% of patients, the margin of the resected specimen was reported as being positive after surgery. Approximately half of the tumors were reported to contain comedo necrosis.
For the primary endpoint, the incidence of invasive breast cancer was reduced by 43% among women assigned to tamoxifen (44 cases-tamoxifen, 74 cases-placebo; p = 0.004; relative risk (RR) = 0.57, 95% CI: 0.39 to 0.84). No data are available regarding the ER status of the invasive cancers. The stage distribution of the invasive cancers at diagnosis was similar to that reported annually in the SEER data base.
Results are shown in Table 1. For each endpoint the following results are presented: the number of events and rate per 1,000 women per year for the placebo and tamoxifen groups; and the relative risk (RR) and its associated 95% confidence interval (CI) between tamoxifen and placebo. Relative risks less than 1.0 indicate a benefit of tamoxifen therapy. The limits of the confidence intervals can be used to assess the statistical significance of the benefits of tamoxifen therapy. If the upper limit of the CI is less than 1.0, then a statistically significant benefit exists.

Survival was similar in the placebo and tamoxifen groups. At 5 years from study entry, survival was 97% for both groups.
Reduction in Breast Cancer Incidence in High Risk Women
The Breast Cancer Prevention Trial (BCPT, NSABP P-1) was a double-blind, randomized, placebo-controlled trial with a primary objective to determine whether 5 years of tamoxifen therapy (20 mg/day) would reduce the incidence of invasive breast cancer in women at high risk for the disease (see INDICATIONS AND USAGE). Secondary objectives included an evaluation of the incidence of ischemic heart disease; the effects on the incidence of bone fractures; and other events that might be associated with the use of tamoxifen, including: endometrial cancer, pulmonary embolus, deep-vein thrombosis, stroke, and cataract formation and surgery (see WARNINGS).
The Gail Model was used to calculate predicted breast cancer risk for women who were less than 60 years of age and did not have lobular carcinoma in situ (LCIS). The following risk factors were used: age; number of first-degree female relatives with breast cancer; previous breast biopsies; presence or absence of atypical hyperplasia; nulliparity; age at first live birth; and age at menarche. A 5 year predicted risk of breast cancer of ≥ 1.67% was required for entry into the trial.
In this trial, 13,388 women of at least 35 years of age were randomized to receive either tamoxifen or placebo for five years. The median duration of treatment was 3.5 years. As of January 31, 1998, follow-up data is available for 13,114 women. Twenty-seven percent of women randomized to placebo (1,782) and 24% of women randomized to tamoxifen (1,596) completed 5 years of therapy. The demographic characteristics of women on the trial with follow-up data are shown in Table 2.
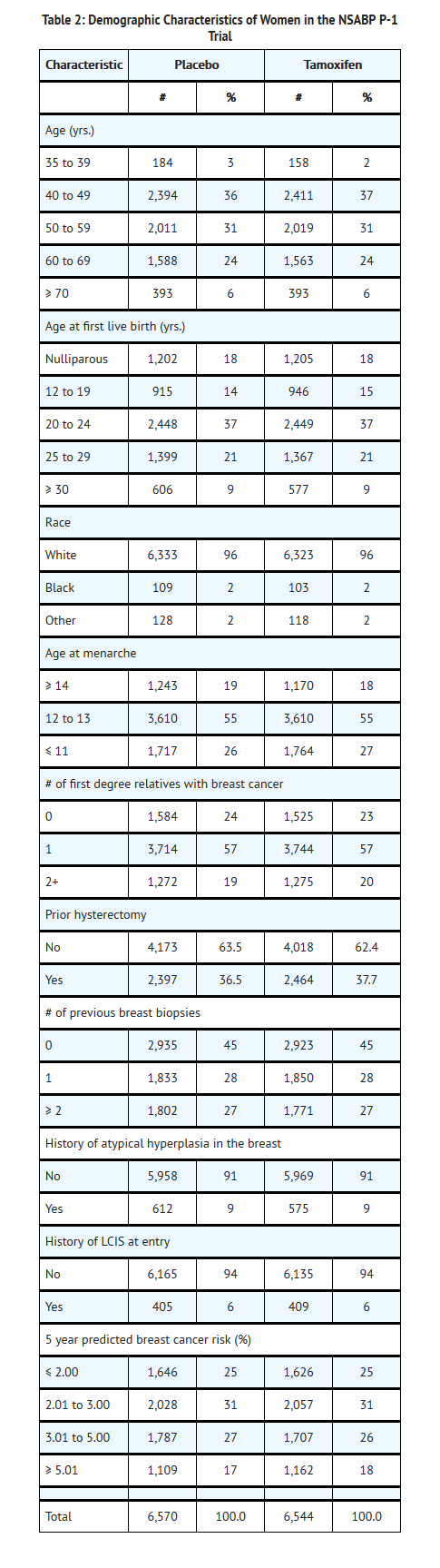
Results are shown in Table 3. After a median follow-up of 4.2 years, the incidence of invasive breast cancer was reduced by 44% among women assigned to tamoxifen (86 cases-tamoxifen, 156 cases-placebo; p < 0.00001; relative risk (RR) = 0.56, 95% CI: 0.43 to 0.72). A reduction in the incidence of breast cancer was seen in each prospectively specified age group (≤ 49, 50 to 59, ≥ 60), in women with or without LCIS, and in each of the absolute risk levels specified in Table 3. A non-significant decrease in the incidence of ductal carcinoma in situ (DCIS) was seen (23 tamoxifen, 35 placebo; RR = 0.66, 95% CI: 0.39 to 1.11).
There was no statistically significant difference in the number of myocardial infarctions, severe angina, or acute ischemic cardiac events between the two groups (61 tamoxifen, 59 placebo; RR = 1.04, 95% CI: 0.73 to 1.49).
No overall difference in mortality (53 deaths in tamoxifen group vs. 65 deaths in placebo group) was present. No difference in breast cancer-related mortality was observed (4 deaths in tamoxifen group vs. 5 deaths in placebo group).
Although there was a non-significant reduction in the number of hip fractures (9 on tamoxifen, 20 on placebo) in the tamoxifen group, the number of wrist fractures was similar in the two treatment groups (69 on tamoxifen, 74 on placebo). A subgroup analysis of the P-1 trial, suggests a difference in effect in bone mineral density (BMD) related to menopausal status in patients receiving tamoxifen. In postmenopausal women there was no evidence of bone loss of the lumbar spine and hip. Conversely, tamoxifen was associated with significant bone loss of the lumbar spine and hip in premenopausal women.
The risks of tamoxifen therapy include endometrial cancer, DVT, PE, stroke, cataract formation, and cataract surgery (see Table 3). In the NSABP P-1 trial, 33 cases of endometrial cancer were observed in the tamoxifen group vs. 14 in the placebo group (RR = 2.48, 95% CI: 1.27 to 4.92). Deep-vein thrombosis was observed in 30 women receiving tamoxifen vs. 19 in women receiving placebo (RR = 1.59, 95% CI: 0.86 to 2.98). Eighteen cases of pulmonary embolism were observed in the tamoxifen group vs. 6 in the placebo group (RR = 3.01, 95% CI: 1.15 to 9.27). There were 34 strokes on the tamoxifen arm and 24 on the placebo arm (RR = 1.42, 95% CI: 0.82 to 2.51). Cataract formation in women without cataracts at baseline was observed in 540 women taking tamoxifen vs. 483 women receiving placebo (RR = 1.13, 95% CI: 1.00 to 1.28). Cataract surgery (with or without cataracts at baseline) was performed in 201 women taking tamoxifen vs. 129 women receiving placebo (RR = 1.51, 95% CI: 1.21 to 1.89) (see WARNINGS).
Table 3 summarizes the major outcomes of the NSABP P-1 trial. For each endpoint, the following results are presented: the number of events and rate per 1,000 women per year for the placebo and tamoxifen groups; and the relative risk (RR) and its associated 95% confidence interval (CI) between tamoxifen and placebo. Relative risks less than 1.0 indicate a benefit of tamoxifen therapy. The limits of the confidence intervals can be used to assess the statistical significance of the benefits or risks of tamoxifen therapy. If the upper limit of the CI is less than 1.0, then a statistically significant benefit exists.
For most participants, multiple risk factors would have been required for eligibility. This table considers risk factors individually, regardless of other co-existing risk factors, for women who developed breast cancer. The 5 year predicted absolute breast cancer risk accounts for multiple risk factors in an individual and should provide the best estimate of individual benefit (see INDICATIONS AND USAGE).
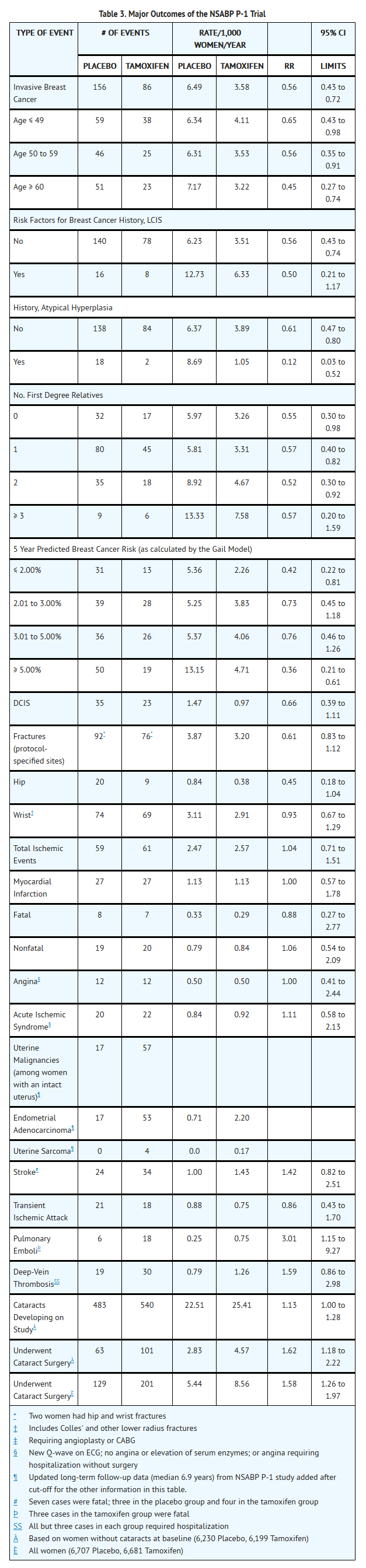
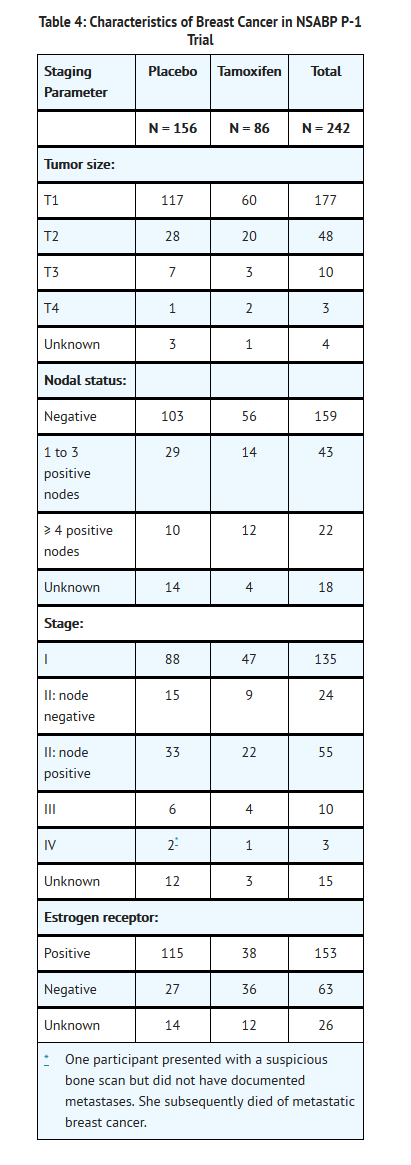
Table 4 describes the characteristics of the breast cancers in the NSABP P-1 trial and includes tumor size, nodal status, ER status. Tamoxifen decreased the incidence of small estrogen receptor positive tumors, but did not alter the incidence of estrogen receptor negative tumors or larger tumors.
Interim results from 2 trials in addition to the NSABP P-1 trial examining the effects of tamoxifen in reducing breast cancer incidence have been reported.
The first was the Italian Tamoxifen Prevention trial. In this trial women between the ages of 35 and 70, who had had a total hysterectomy, were randomized to receive 20 mg tamoxifen or matching placebo for 5 years. The primary endpoints were occurrence of, and death from, invasive breast cancer. Women without any specific risk factors for breast cancer were to be entered. Between 1992 and 1997, 5,408 women were randomized. Hormone Replacement Therapy (HRT) was used in 14% of participants. The trial closed in 1997 due to the large number of dropouts during the first year of treatment (26%). After 46 months of follow-up there were 22 breast cancers in women on placebo and 19 in women on tamoxifen. Although no decrease in breast cancer incidence was observed, there was a trend for reduction in breast cancer among women receiving protocol therapy for at least 1 year (19 placebo, 11 tamoxifen). The small numbers of participants along with the low level of risk in this otherwise healthy group precluded an adequate assessment of the effect of tamoxifen in reducing the incidence of breast cancer.
The second trial, the Royal Marsden Trial (RMT) was reported as an interim analysis. The RMT was begun in 1986 as a feasibility study of whether larger scale trials could be mounted. The trial was subsequently extended to a pilot trial to accrue additional participants to further assess the safety of tamoxifen. Twenty-four hundred and seventy-one women were entered between 1986 and 1996; they were selected on the basis of a family history of breast cancer. HRT was used in 40% of participants. In this trial, with a 70 month median follow-up, 34 and 36 breast cancers (8 noninvasive, 4 on each arm) were observed among women on tamoxifen and placebo, respectively. Patients in this trial were younger than those in the NSABP P-1 trial and may have been more likely to develop ER (-) tumors, which are unlikely to be reduced in number by tamoxifen therapy. Although women were selected on the basis of family history and were thought to have a high risk of breast cancer, few events occurred, reducing the statistical power of the study. These factors are potential reasons why the RMT may not have provided an adequate assessment of the effectiveness of tamoxifen in reducing the incidence of breast cancer.
In these trials, an increased number of cases of deep-vein thrombosis, pulmonary embolus, stroke, and endometrial cancer were observed on the tamoxifen arm compared to the placebo arm. The frequency of events was consistent with the safety data observed in the NSABP P-1 trial.
McCune-Albright Syndrome
A single, uncontrolled multicenter trial of tamoxifen 20 mg once a day was conducted in a heterogenous group of girls with McCune-Albright syndrome and precocious puberty manifested by physical signs of pubertal development, episodes of vaginal bleeding and/or advanced bone age (bone age of at least 12 months beyond chronological age). Twenty-eight female pediatric patients, aged 2 to 10 years, were treated for up to 12 months. Effect of treatment on frequency of vaginal bleeding, bone age advancement, and linear growth rate was assessed relative to prestudy baseline. Tamoxifen treatment was associated with a 50% reduction in frequency of vaginal bleeding episodes by patient or family report (mean annualized frequency of 3.56 episodes at baseline and 1.73 episodes on-treatment). Among the patients who reported vaginal bleeding during the prestudy period, 62% (13 out of 21 patients) reported no bleeding for a 6 month period and 33% (7 out of 21 patients) reported no vaginal bleeding for the duration of the trial. Not all patients improved on treatment and a few patients not reporting vaginal bleeding in the 6 months prior to enrollment reported menses on treatment. Tamoxifen therapy was associated with a reduction in mean rate of increase of bone age. Individual responses with regard to bone age advancement were highly heterogeneous. Linear growth rate was reduced during the course of tamoxifen treatment in a majority of patients (mean change of 1.68 cm/year relative to baseline; change from 7.47 cm/year at baseline to 5.79 cm/year on study). This change was not uniformly seen across all stages of bone maturity; all recorded response failures occurred in patients with bone ages less than 7 years at screening.
Mean uterine volume increased after 6 months of treatment and doubled at the end of the one-year study. A causal relationship has not been established; however, as an increase in the incidence of endometrial adenocarcinoma and uterine sarcoma has been noted in adults treated with tamoxifen (see BOXED WARNING), continued monitoring of McCune-Albright patients treated with tamoxifen for long-term uterine effects is recommended. The safety and efficacy of tamoxifen for girls aged 2 to 10 years with McCune-Albright syndrome and precocious puberty have not been studied beyond one year of treatment. The long-term effects of tamoxifen therapy in girls have not been established.
How Supplied
- Tamoxifen citrate tablets USP, 10 mg (base) are white, round, biconvex, film-coated, unscored tablets debossed “93” and “784” and are supplied in bottles of 30, 50, 60, 90 and 100.
Storage
- Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
- Dispense in a well-closed, light-resistant container as defined in the USP, with a child-resistant closure (as required).
Images
Drug Images
{{#ask: Page Name::Tamoxifen |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Tamoxifen |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information

Precautions with Alcohol
- Alcohol-Tamoxifen interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Nolvadex, Soltamox.
Look-Alike Drug Names
- A® — B®[1]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Tamoxifen
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Tamoxifen |Label Name=Tamoxifen11.png
}}
{{#subobject:
|Label Page=Tamoxifen |Label Name=Tamoxifen11.png
}}