Sulfadiazine: Difference between revisions
No edit summary |
No edit summary |
||
| Line 43: | Line 43: | ||
====Toxoplasma encephalitis==== | ====Toxoplasma encephalitis==== | ||
====Trachoma==== | ====Trachoma==== | ||
| Line 102: | Line 101: | ||
====Haemophilus influenzae meningitis, In combination with parenteral streptomycin; Adjunct==== | ====Haemophilus influenzae meningitis, In combination with parenteral streptomycin; Adjunct==== | ||
====Inclusion conjunctivitis==== | |||
====Malaria, due to chloroquine-resistant strains of Plasmodium falciparum; Adjunct==== | |||
====Meningococcal meningitis; Treatment and Prophylaxis==== | |||
====Nocardiosis==== | |||
====Rheumatic fever, Recurrent; Prophylaxis==== | |||
====Toxoplasma encephalitis==== | |||
Toxoplasma encephalitis | |||
====Trachoma==== | |||
====Urinary tract infectious disease==== | |||
<!--Off-Label Use and Dosage (Pediatric)--> | <!--Off-Label Use and Dosage (Pediatric)--> | ||
| Line 153: | Line 148: | ||
<!--Contraindications--> | <!--Contraindications--> | ||
|contraindications=Sulfadiazine is contraindicated in the following circumstances: Hypersensitivity to sulfonamides. | |contraindications=*Sulfadiazine is contraindicated in the following circumstances: Hypersensitivity to sulfonamides. | ||
In infants less than 2 months of age (except as adjunctive therapy with pyrimethamine in the treatment of congenital toxoplasmosis). | *In infants less than 2 months of age (except as adjunctive therapy with pyrimethamine in the treatment of congenital toxoplasmosis). | ||
In pregnancy at term and during the nursing period, because sulfonamides cross the placenta and are excreted in breast milk and may cause kernicterus. | *In pregnancy at term and during the nursing period, because sulfonamides cross the placenta and are excreted in breast milk and may cause kernicterus. | ||
|warnings=The sulfonamides should not be used for the treatment of group A betahemolytic streptococcal infections. In an established infection, they will not eradicate the streptococcus and, therefore, will not prevent sequelae such as rheumatic fever and glomerulonephritis. | |warnings=*The sulfonamides should not be used for the treatment of group A betahemolytic streptococcal infections. In an established infection, they will not eradicate the streptococcus and, therefore, will not prevent sequelae such as rheumatic fever and glomerulonephritis. | ||
Deaths associated with the administration of sulfonamides have been reported from hypersensitivity reactions, agranulocytosis, aplastic anemia and other blood dyscrasias. | *Deaths associated with the administration of sulfonamides have been reported from hypersensitivity reactions, agranulocytosis, aplastic anemia and other blood dyscrasias. | ||
The presence of such clinical signs as sore throat, fever, pallor, purpura or jaundice may be early indications of serious blood disorders. | *The presence of such clinical signs as sore throat, fever, pallor, purpura or jaundice may be early indications of serious blood disorders. | ||
The frequency of renal complications is considerably lower in patients receiving the more soluble sulfonamides. | *The frequency of renal complications is considerably lower in patients receiving the more soluble sulfonamides. | ||
====Precautions==== | ====Precautions==== | ||
| Line 170: | Line 165: | ||
'''General''' | '''General''' | ||
Sulfonamides should be given with caution to patients with impaired renal or hepatic function and to those with severe allergy or bronchial asthma. | *Sulfonamides should be given with caution to patients with impaired renal or hepatic function and to those with severe allergy or bronchial asthma. | ||
Hemolysis may occur in individuals deficient in glucose-6-phosphate dehydrogenase. This reaction is dose related. | *Hemolysis may occur in individuals deficient in glucose-6-phosphate dehydrogenase. This reaction is dose related. | ||
Adequate fluid intake must be maintained in order to prevent crystalluria and stone formation. | *Adequate fluid intake must be maintained in order to prevent crystalluria and stone formation. | ||
Laboratory Tests | '''Laboratory Tests''' | ||
Complete blood counts and urinalyses with careful microscopic examinations should be done frequently in patients receiving sulfonamides. | *Complete blood counts and urinalyses with careful microscopic examinations should be done frequently in patients receiving sulfonamides. | ||
<!--Adverse Reactions--> | <!--Adverse Reactions--> | ||
<!--Clinical Trials Experience--> | <!--Clinical Trials Experience--> | ||
|clinicalTrials=Blood Dyscrasias | |clinicalTrials='''Blood Dyscrasias''' | ||
*Agranulocytosis, aplastic anemia, thrombocytopenia, leukopenia, hemolytic anemia, purpura, hypoprothrombinemia and methemoglobinemia. | |||
'''Allergic Reactions''' | |||
*Erythema multiforme (Stevens-Johnson syndrome), generalized skin eruptions, epidermal necrolysis, urticaria, serum sickness, pruritus, exfoliative dermatitis, anaphylactoid reactions, periorbital edema, conjunctival and scleral injection, photosensitization, arthralgia, allergic myocarditis, drug fever and chills. | |||
'''Gastrointestinal Reactions''' | |||
*Nausea, emesis, abdominal pains, hepatitis, diarrhea, anorexia, pancreatitis and stomatitis. | |||
'''C.N.S. Reactions''' | |||
*Headache, peripheral neuritis, mental depression, convulsions, ataxia, hallucinations, tinnitus, vertigo and insomnia. | |||
'''Renal''' | |||
*Crystalluria, stone formation, toxic nephrosis with oliguria and anuria; periarteritis nodosa and lupus erythematosus phenomenon have been noted. | |||
'''Miscellaneous Reactions''' | |||
*The sulfonamides bear certain chemical similarities to some goitrogens, diuretics (acetazolamide and the thiazides) and oral hypoglycemic agents. Goiter production, diuresis, and hypoglycemia have occurred rarely in patients receiving sulfonamides. Cross-sensitivity may exist with these agents. | |||
|postmarketing=* There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |||
<!--Drug Interactions--> | <!--Drug Interactions--> | ||
|drugInteractions=Administration of a sulfonamide may increase the effect of oral anticoagulants and methotrexate, probably by displacement of these drugs from binding sites on plasma albumin. Potentiation of the action of sulfonylurea hypoglycemic agents, thiazide diuretics and uricosuric agents may also be noted. This may also be due to displacement of the drugs from albumin or a pharmacodynamic mechanism may play a role. Conversely, agents such as indomethacin, probenecid and salicylates may displace sulfonamides from plasma albumin and increase the concentrations of free drug in plasma. | |drugInteractions=* Administration of a sulfonamide may increase the effect of oral anticoagulants and methotrexate, probably by displacement of these drugs from binding sites on plasma albumin. Potentiation of the action of sulfonylurea hypoglycemic agents, thiazide diuretics and uricosuric agents may also be noted. This may also be due to displacement of the drugs from albumin or a pharmacodynamic mechanism may play a role. Conversely, agents such as indomethacin, probenecid and salicylates may displace sulfonamides from plasma albumin and increase the concentrations of free drug in plasma. | ||
<!--Use in Specific Populations--> | <!--Use in Specific Populations--> | ||
|FDAPregCat=C | |FDAPregCat=C | ||
|useInPregnancyFDA=Teratogenic Effects | |useInPregnancyFDA='''Teratogenic Effects''' | ||
Pregnancy Category C | '''Pregnancy Category C''' | ||
The safe use of sulfonamides in pregnancy has not been established. The teratogenic potential of most sulfonamides has not been thoroughly investigated in either animals or humans. However, a significant increase in the incidence of cleft palate and other bony abnormalities in offspring has been observed when certain sulfonamides of the short, intermediate and long acting types were given to pregnant rats and mice in high oral doses (7 to 25 times the human therapeutic dose). | *The safe use of sulfonamides in pregnancy has not been established. The teratogenic potential of most sulfonamides has not been thoroughly investigated in either animals or humans. However, a significant increase in the incidence of cleft palate and other bony abnormalities in offspring has been observed when certain sulfonamides of the short, intermediate and long acting types were given to pregnant rats and mice in high oral doses (7 to 25 times the human therapeutic dose). | ||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | ||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | *There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | ||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |useInLaborDelivery=*There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | ||
|useInNursing=Sulfadiazine is contraindicated for use in nursing mothers because the sulfonamides cross the placenta, are excreted in breast milk and may cause kernicterus. | |useInNursing=*Sulfadiazine is contraindicated for use in nursing mothers because the sulfonamides cross the placenta, are excreted in breast milk and may cause kernicterus. | ||
Because of the potential for serious adverse reactions in nursing infants from sulfadiazine, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. See CONTRAINDICATIONS. | *Because of the potential for serious adverse reactions in nursing infants from sulfadiazine, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. See CONTRAINDICATIONS. | ||
|useInPed=Sulfadiazine is contraindicated in infants less than 2 months of age (except as adjunctive therapy with pyrimethamine in the treatment of congenital toxoplasmosis). See CONTRAINDICATIONSand DOSAGE AND ADMINISTRATION. | |useInPed=*Sulfadiazine is contraindicated in infants less than 2 months of age (except as adjunctive therapy with pyrimethamine in the treatment of congenital toxoplasmosis). See CONTRAINDICATIONSand DOSAGE AND ADMINISTRATION. | ||
|useInGeri=There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | |useInGeri=*There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | ||
|useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |useInGender=*There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | ||
|useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |useInRace=*There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | ||
|useInRenalImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |useInRenalImpair=*There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | ||
|useInHepaticImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | |useInHepaticImpair=*There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | ||
|useInReproPotential=There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |useInReproPotential=*There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | ||
|useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |useInImmunocomp=*There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | ||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration=SYSTEMIC SULFONAMIDES ARE CONTRAINDICATED IN INFANTS UNDER 2 MONTHS OF AGE except as adjunctive therapy with pyrimethamine in the treatment of congenital toxoplasmosis. | |administration=*SYSTEMIC SULFONAMIDES ARE CONTRAINDICATED IN INFANTS UNDER 2 MONTHS OF AGE except as adjunctive therapy with pyrimethamine in the treatment of congenital toxoplasmosis. | ||
Usual Dosage for Infants over 2 Months of Age and Children | *Usual Dosage for Infants over 2 Months of Age and Children | ||
Initially, one-half the 24-hour dose. Maintenance, 150 mg/kg or 4 g/m2, divided into 4 to 6 doses, every 24 hours, with a maximum of 6 g every 24 hours. Rheumatic fever prophylaxis, under 30 kg (66 pounds), 500 mg every 24 hours; over 30 kg (66 pounds), 1 g every 24 hours. | *Initially, one-half the 24-hour dose. Maintenance, 150 mg/kg or 4 g/m2, divided into 4 to 6 doses, every 24 hours, with a maximum of 6 g every 24 hours. Rheumatic fever prophylaxis, under 30 kg (66 pounds), 500 mg every 24 hours; over 30 kg (66 pounds), 1 g every 24 hours. | ||
Usual Adult Dosage | '''Usual Adult Dosage''' | ||
Initially, 2 g to 4 g. Maintenance, 2 g to 4 g, divided into 3 to 6 doses, every 24 hours. | *Initially, 2 g to 4 g. Maintenance, 2 g to 4 g, divided into 3 to 6 doses, every 24 hours. | ||
|monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |monitoring=*There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | ||
<!--IV Compatibility--> | <!--IV Compatibility--> | ||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |IVCompat=*There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | ||
<!--Overdosage--> | <!--Overdosage--> | ||
|overdose= | |overdose=* There is limited information regarding <i>Chronic Overdose</i> of {{PAGENAME}} in the drug label. | ||
* | |||
There is limited information regarding <i>Chronic Overdose</i> of {{PAGENAME}} in the drug label. | |||
<!--Pharmacology--> | <!--Pharmacology--> | ||
| Line 323: | Line 252: | ||
|drugBox=[[File:Sulfadiazine wiki.png|600px|thumbnail|left]] | |drugBox=[[File:Sulfadiazine wiki.png|600px|thumbnail|left]] | ||
{{clear}} | {{clear}} | ||
|mechAction=The systemic sulfonamides are bacteriostatic agents having a similar spectrum of activity. Sulfonamides competitively inhibit bacterial synthesis of folic acid (pteroylglutamic acid) from aminobenzoic acid. Resistant strains are capable of utilizing folic acid precursors or preformed folic acid. | |mechAction=* The systemic sulfonamides are bacteriostatic agents having a similar spectrum of activity. Sulfonamides competitively inhibit bacterial synthesis of folic acid (pteroylglutamic acid) from aminobenzoic acid. Resistant strains are capable of utilizing folic acid precursors or preformed folic acid. | ||
|structure=Sulfadiazine is an oral sulfonamide anti-bacterial agent. | |structure=*Sulfadiazine is an oral sulfonamide anti-bacterial agent. | ||
Each tablet, for oral administration, contains 500 mg sulfadiazine. In addition, each tablet contains the following inactive ingredients: croscarmellose sodium, docusate sodium, microcrystalline cellulose, povidone, sodium benzoate, sodium starch glycolate and stearic acid. | *Each tablet, for oral administration, contains 500 mg sulfadiazine. In addition, each tablet contains the following inactive ingredients: croscarmellose sodium, docusate sodium, microcrystalline cellulose, povidone, sodium benzoate, sodium starch glycolate and stearic acid. | ||
Sulfadiazine occurs as a white or slightly yellow powder. It is odorless or nearly so and slowly darkens on exposure to light. It is practically insoluble in water and slightly soluble in alcohol. The chemical name of sulfadiazine is N1-2-pyrimidinylsulfanilamide. The molecular formula is C10H10N4O2S. It has a molecular weight of 250.27. The structural formula is shown below: | *Sulfadiazine occurs as a white or slightly yellow powder. It is odorless or nearly so and slowly darkens on exposure to light. It is practically insoluble in water and slightly soluble in alcohol. The chemical name of sulfadiazine is N1-2-pyrimidinylsulfanilamide. The molecular formula is C10H10N4O2S. It has a molecular weight of 250.27. The structural formula is shown below: | ||
[[File:Sulfadiazine structure.jpg|600px|thumbnail|left]] | [[File:Sulfadiazine structure.jpg|600px|thumbnail|left]] | ||
{{clear}} | {{clear}} | ||
Most sulfonamides slowly darken on exposure to light. | *Most sulfonamides slowly darken on exposure to light. | ||
|PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |PD=*There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | ||
<!--Pharmacokinetics--> | <!--Pharmacokinetics--> | ||
|PK=Sulfonamides exist in the blood in 3 forms - free, conjugated (acetylated and possibly others) and protein bound. The free form is considered to be the therapeutically active one. | |PK=*Sulfonamides exist in the blood in 3 forms - free, conjugated (acetylated and possibly others) and protein bound. The free form is considered to be the therapeutically active one. | ||
Sulfadiazine given orally is readily absorbed from the gastrointestinal tract. After a single 2 g oral dose, a peak of 6.04 mg/100 mL is reached in 4 hours; of this, 4.65 mg/100 mL is free drug. | *Sulfadiazine given orally is readily absorbed from the gastrointestinal tract. After a single 2 g oral dose, a peak of 6.04 mg/100 mL is reached in 4 hours; of this, 4.65 mg/100 mL is free drug. | ||
When a dose of 100 mg/kg of body weight is given initially and followed by 50 mg/kg every 6 hours, blood levels of free sulfadiazine are about 7 mg/100mL. Protein binding is 38% to 48%. Sulfadiazine diffuses into the cerebrospinal fluid; free drug reaches 32% to 65% of blood levels and total drug 40% to 60%. | *When a dose of 100 mg/kg of body weight is given initially and followed by 50 mg/kg every 6 hours, blood levels of free sulfadiazine are about 7 mg/100mL. Protein binding is 38% to 48%. Sulfadiazine diffuses into the cerebrospinal fluid; free drug reaches 32% to 65% of blood levels and total drug 40% to 60%. | ||
Sulfadiazine is excreted largely in the urine, where concentrations are 10 to 25 times greater than serum levels. Approximately 10% of a single oral dose is excreted in the first 6 hours, 50% within 24 hours and 60% to 85% in 48 to 72 hours. Of the amount excreted in the urine, 15% to 40% is in the acetyl form. | *Sulfadiazine is excreted largely in the urine, where concentrations are 10 to 25 times greater than serum levels. Approximately 10% of a single oral dose is excreted in the first 6 hours, 50% within 24 hours and 60% to 85% in 48 to 72 hours. Of the amount excreted in the urine, 15% to 40% is in the acetyl form. | ||
|nonClinToxic=Carcinogenesis, Mutagenesis, Impairment of Fertility | |nonClinToxic='''Carcinogenesis, Mutagenesis, Impairment of Fertility''' | ||
The sulfonamides bear certain chemical similarities to some goitrogens. Rats appear to be especially susceptible to the goitrogenic effects of sulfonamides and long-term administration has produced thyroid malignancies in rats. | *The sulfonamides bear certain chemical similarities to some goitrogens. Rats appear to be especially susceptible to the goitrogenic effects of sulfonamides and long-term administration has produced thyroid malignancies in rats. | ||
|clinicalStudies=There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | |clinicalStudies=*There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | ||
<!--How Supplied--> | <!--How Supplied--> | ||
|howSupplied=*SulfADIAZine Tablets USP for oral administration are available as: | |howSupplied=*SulfADIAZine Tablets USP for oral administration are available as: | ||
500 mg: white, unscored, capsule-shaped tablets, debossed “E 757” on one face and supplied as: | *500 mg: white, unscored, capsule-shaped tablets, debossed “E 757” on one face and supplied as: | ||
NDC 0185-0757-30 bottles of 30 | *NDC 0185-0757-30 bottles of 30 | ||
NDC 0185-0757-01 bottles of 100 | *NDC 0185-0757-01 bottles of 100 | ||
NDC 0185-0757-10 bottles of 1000 | *NDC 0185-0757-10 bottles of 1000 | ||
|storage=Storage: Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. | |storage=* Storage: Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. | ||
Dispense in a tight, light-resistant container as defined in the USP with a child-resistant closure, as required. | * Dispense in a tight, light-resistant container as defined in the USP with a child-resistant closure, as required. | ||
To report SUSPECTED ADVERSE REACTIONS, contact Sandoz Inc. at 1-800-525-8747 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. | * To report SUSPECTED ADVERSE REACTIONS, contact Sandoz Inc. at 1-800-525-8747 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. | ||
|packLabel=[[File:Sulfadiazine pdp.jpg|600px|thumbnail|left]] | |packLabel=[[File:Sulfadiazine pdp.jpg|600px|thumbnail|left]] | ||
{{clear}} | {{clear}} | ||
| Line 369: | Line 298: | ||
[[File:Sulfadiazine label.png|600px|thumbnail|left]] | [[File:Sulfadiazine label.png|600px|thumbnail|left]] | ||
{{clear}} | {{clear}} | ||
|fdaPatientInfo=Information for Patients | |fdaPatientInfo='''Information for Patients''' | ||
Patients should be instructed to drink an eight ounce glass of water with each dose of medication and at frequent intervals throughout the day. Caution patients to report promptly the onset of sore throat, fever, pallor, purpura or jaundice when taking this drug, since these may be early indications of serious blood disorders. | *Patients should be instructed to drink an eight ounce glass of water with each dose of medication and at frequent intervals throughout the day. Caution patients to report promptly the onset of sore throat, fever, pallor, purpura or jaundice when taking this drug, since these may be early indications of serious blood disorders. | ||
|alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
Revision as of 16:36, 22 January 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Deepika Beereddy, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Sulfadiazine is a anti-infective, anti-parasitic agent that is FDA approved for the treatment of {{{indication}}}. Common adverse reactions include .
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Acute otitis media, Due to Haemophilus influenzae, in combination with penicillin
- Dosing Information
- Dosage
Chancroid
- Dosing Information
- Dosage
Haemophilus influenzae meningitis, In combination with parenteral streptomycin; Adjunct
- Dosing Information
- Dosage
Inclusion conjunctivitis
Malaria, due to chloroquine-resistant strains of Plasmodium falciparum; Adjunct
Meningococcal meningitis; Treatment and Prophylaxis
Nocardiosis
Rheumatic fever, Recurrent; Prophylaxis
Toxoplasma encephalitis
Trachoma
Urinary tract infectious disease
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Congenital toxoplasmosis
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Sulfadiazine in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sulfadiazine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Acute otitis media, Due to Haemophilus influenzae, in combination with penicillin
- Dosing Information
- Dosage
Chancroid
- Dosing Information
- Dosage
Congenital toxoplasmosis; Adjunct
- Dosing Information
- Dosage
Haemophilus influenzae meningitis, In combination with parenteral streptomycin; Adjunct
Inclusion conjunctivitis
Malaria, due to chloroquine-resistant strains of Plasmodium falciparum; Adjunct
Meningococcal meningitis; Treatment and Prophylaxis
Nocardiosis
Rheumatic fever, Recurrent; Prophylaxis
Toxoplasma encephalitis
Trachoma
Urinary tract infectious disease
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Sulfadiazine in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sulfadiazine in pediatric patients.
Contraindications
- Sulfadiazine is contraindicated in the following circumstances: Hypersensitivity to sulfonamides.
- In infants less than 2 months of age (except as adjunctive therapy with pyrimethamine in the treatment of congenital toxoplasmosis).
- In pregnancy at term and during the nursing period, because sulfonamides cross the placenta and are excreted in breast milk and may cause kernicterus.
Warnings
- The sulfonamides should not be used for the treatment of group A betahemolytic streptococcal infections. In an established infection, they will not eradicate the streptococcus and, therefore, will not prevent sequelae such as rheumatic fever and glomerulonephritis.
- Deaths associated with the administration of sulfonamides have been reported from hypersensitivity reactions, agranulocytosis, aplastic anemia and other blood dyscrasias.
- The presence of such clinical signs as sore throat, fever, pallor, purpura or jaundice may be early indications of serious blood disorders.
- The frequency of renal complications is considerably lower in patients receiving the more soluble sulfonamides.
Precautions
General
- Sulfonamides should be given with caution to patients with impaired renal or hepatic function and to those with severe allergy or bronchial asthma.
- Hemolysis may occur in individuals deficient in glucose-6-phosphate dehydrogenase. This reaction is dose related.
- Adequate fluid intake must be maintained in order to prevent crystalluria and stone formation.
Laboratory Tests
- Complete blood counts and urinalyses with careful microscopic examinations should be done frequently in patients receiving sulfonamides.
Adverse Reactions
Clinical Trials Experience
Blood Dyscrasias
- Agranulocytosis, aplastic anemia, thrombocytopenia, leukopenia, hemolytic anemia, purpura, hypoprothrombinemia and methemoglobinemia.
Allergic Reactions
- Erythema multiforme (Stevens-Johnson syndrome), generalized skin eruptions, epidermal necrolysis, urticaria, serum sickness, pruritus, exfoliative dermatitis, anaphylactoid reactions, periorbital edema, conjunctival and scleral injection, photosensitization, arthralgia, allergic myocarditis, drug fever and chills.
Gastrointestinal Reactions
- Nausea, emesis, abdominal pains, hepatitis, diarrhea, anorexia, pancreatitis and stomatitis.
C.N.S. Reactions
- Headache, peripheral neuritis, mental depression, convulsions, ataxia, hallucinations, tinnitus, vertigo and insomnia.
Renal
- Crystalluria, stone formation, toxic nephrosis with oliguria and anuria; periarteritis nodosa and lupus erythematosus phenomenon have been noted.
Miscellaneous Reactions
- The sulfonamides bear certain chemical similarities to some goitrogens, diuretics (acetazolamide and the thiazides) and oral hypoglycemic agents. Goiter production, diuresis, and hypoglycemia have occurred rarely in patients receiving sulfonamides. Cross-sensitivity may exist with these agents.
Postmarketing Experience
- There is limited information regarding Postmarketing Experience of Sulfadiazine in the drug label.
Drug Interactions
- Administration of a sulfonamide may increase the effect of oral anticoagulants and methotrexate, probably by displacement of these drugs from binding sites on plasma albumin. Potentiation of the action of sulfonylurea hypoglycemic agents, thiazide diuretics and uricosuric agents may also be noted. This may also be due to displacement of the drugs from albumin or a pharmacodynamic mechanism may play a role. Conversely, agents such as indomethacin, probenecid and salicylates may displace sulfonamides from plasma albumin and increase the concentrations of free drug in plasma.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C Teratogenic Effects
Pregnancy Category C
- The safe use of sulfonamides in pregnancy has not been established. The teratogenic potential of most sulfonamides has not been thoroughly investigated in either animals or humans. However, a significant increase in the incidence of cleft palate and other bony abnormalities in offspring has been observed when certain sulfonamides of the short, intermediate and long acting types were given to pregnant rats and mice in high oral doses (7 to 25 times the human therapeutic dose).
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
- There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Sulfadiazine in women who are pregnant.
Labor and Delivery
- There is no FDA guidance on use of Sulfadiazine during labor and delivery.
Nursing Mothers
- Sulfadiazine is contraindicated for use in nursing mothers because the sulfonamides cross the placenta, are excreted in breast milk and may cause kernicterus.
- Because of the potential for serious adverse reactions in nursing infants from sulfadiazine, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. See CONTRAINDICATIONS.
Pediatric Use
- Sulfadiazine is contraindicated in infants less than 2 months of age (except as adjunctive therapy with pyrimethamine in the treatment of congenital toxoplasmosis). See CONTRAINDICATIONSand DOSAGE AND ADMINISTRATION.
Geriatic Use
- There is no FDA guidance on the use of Sulfadiazine with respect to geriatric patients.
Gender
- There is no FDA guidance on the use of Sulfadiazine with respect to specific gender populations.
Race
- There is no FDA guidance on the use of Sulfadiazine with respect to specific racial populations.
Renal Impairment
- There is no FDA guidance on the use of Sulfadiazine in patients with renal impairment.
Hepatic Impairment
- There is no FDA guidance on the use of Sulfadiazine in patients with hepatic impairment.
Females of Reproductive Potential and Males
- There is no FDA guidance on the use of Sulfadiazine in women of reproductive potentials and males.
Immunocompromised Patients
- There is no FDA guidance one the use of Sulfadiazine in patients who are immunocompromised.
Administration and Monitoring
Administration
- SYSTEMIC SULFONAMIDES ARE CONTRAINDICATED IN INFANTS UNDER 2 MONTHS OF AGE except as adjunctive therapy with pyrimethamine in the treatment of congenital toxoplasmosis.
- Usual Dosage for Infants over 2 Months of Age and Children
- Initially, one-half the 24-hour dose. Maintenance, 150 mg/kg or 4 g/m2, divided into 4 to 6 doses, every 24 hours, with a maximum of 6 g every 24 hours. Rheumatic fever prophylaxis, under 30 kg (66 pounds), 500 mg every 24 hours; over 30 kg (66 pounds), 1 g every 24 hours.
Usual Adult Dosage
- Initially, 2 g to 4 g. Maintenance, 2 g to 4 g, divided into 3 to 6 doses, every 24 hours.
Monitoring
- There is limited information regarding Monitoring of Sulfadiazine in the drug label.
IV Compatibility
- There is limited information regarding IV Compatibility of Sulfadiazine in the drug label.
Overdosage
- There is limited information regarding Chronic Overdose of Sulfadiazine in the drug label.
Pharmacology
Mechanism of Action
- The systemic sulfonamides are bacteriostatic agents having a similar spectrum of activity. Sulfonamides competitively inhibit bacterial synthesis of folic acid (pteroylglutamic acid) from aminobenzoic acid. Resistant strains are capable of utilizing folic acid precursors or preformed folic acid.
Structure
- Sulfadiazine is an oral sulfonamide anti-bacterial agent.
- Each tablet, for oral administration, contains 500 mg sulfadiazine. In addition, each tablet contains the following inactive ingredients: croscarmellose sodium, docusate sodium, microcrystalline cellulose, povidone, sodium benzoate, sodium starch glycolate and stearic acid.
- Sulfadiazine occurs as a white or slightly yellow powder. It is odorless or nearly so and slowly darkens on exposure to light. It is practically insoluble in water and slightly soluble in alcohol. The chemical name of sulfadiazine is N1-2-pyrimidinylsulfanilamide. The molecular formula is C10H10N4O2S. It has a molecular weight of 250.27. The structural formula is shown below:
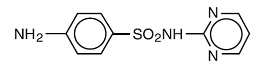
- Most sulfonamides slowly darken on exposure to light.
Pharmacodynamics
- There is limited information regarding Pharmacodynamics of Sulfadiazine in the drug label.
Pharmacokinetics
- Sulfonamides exist in the blood in 3 forms - free, conjugated (acetylated and possibly others) and protein bound. The free form is considered to be the therapeutically active one.
- Sulfadiazine given orally is readily absorbed from the gastrointestinal tract. After a single 2 g oral dose, a peak of 6.04 mg/100 mL is reached in 4 hours; of this, 4.65 mg/100 mL is free drug.
- When a dose of 100 mg/kg of body weight is given initially and followed by 50 mg/kg every 6 hours, blood levels of free sulfadiazine are about 7 mg/100mL. Protein binding is 38% to 48%. Sulfadiazine diffuses into the cerebrospinal fluid; free drug reaches 32% to 65% of blood levels and total drug 40% to 60%.
- Sulfadiazine is excreted largely in the urine, where concentrations are 10 to 25 times greater than serum levels. Approximately 10% of a single oral dose is excreted in the first 6 hours, 50% within 24 hours and 60% to 85% in 48 to 72 hours. Of the amount excreted in the urine, 15% to 40% is in the acetyl form.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- The sulfonamides bear certain chemical similarities to some goitrogens. Rats appear to be especially susceptible to the goitrogenic effects of sulfonamides and long-term administration has produced thyroid malignancies in rats.
Clinical Studies
- There is limited information regarding Clinical Studies of Sulfadiazine in the drug label.
How Supplied
- SulfADIAZine Tablets USP for oral administration are available as:
- 500 mg: white, unscored, capsule-shaped tablets, debossed “E 757” on one face and supplied as:
- NDC 0185-0757-30 bottles of 30
- NDC 0185-0757-01 bottles of 100
- NDC 0185-0757-10 bottles of 1000
Storage
- Storage: Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
- Dispense in a tight, light-resistant container as defined in the USP with a child-resistant closure, as required.
- To report SUSPECTED ADVERSE REACTIONS, contact Sandoz Inc. at 1-800-525-8747 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Images
Drug Images
{{#ask: Page Name::Sulfadiazine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

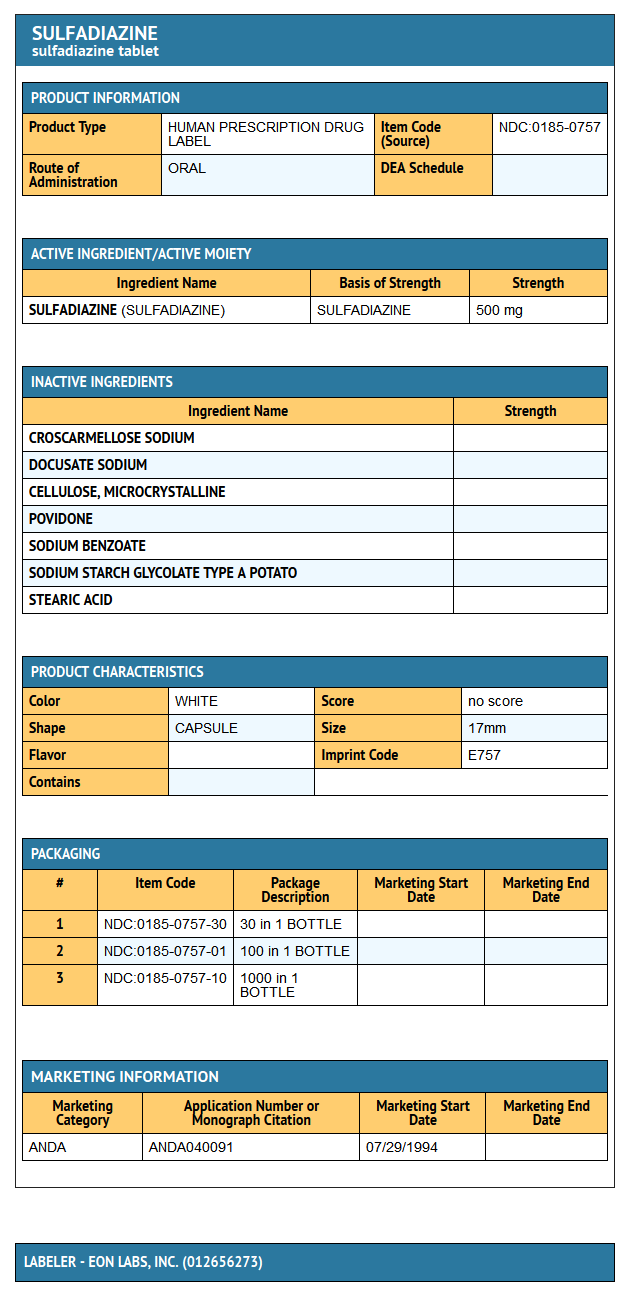
{{#ask: Label Page::Sulfadiazine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Information for Patients
- Patients should be instructed to drink an eight ounce glass of water with each dose of medication and at frequent intervals throughout the day. Caution patients to report promptly the onset of sore throat, fever, pallor, purpura or jaundice when taking this drug, since these may be early indications of serious blood disorders.
Precautions with Alcohol
- Alcohol-Sulfadiazine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Sulfadiazine
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Sulfadiazine |Label Name=Sulfadiazine11.png
}}
{{#subobject:
|Label Page=Sulfadiazine |Label Name=Sulfadiazine11.png
}}