St. Louis encephalitis pathophysiology: Difference between revisions
m (Changes made per Mahshid's request) |
|||
| (16 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{St. Louis encephalitis}} | {{St. Louis encephalitis}} | ||
{{CMG}} {{AE}}: {{VVS}} | {{CMG}}; {{AE}} {{AG}}; '''Contributor(s):''' {{Irfan Dotani}}, {{VVS}} | ||
== | ==Overview== | ||
[[ | St. Louis encephalitis virus is usually transmitted via [[mosquito]]s (generally from the genus ''[[Culex]]'') to the human host. St. Louis encephalitis virus contains [[positive-sense ssRNA virus|positive-sense]] viral [[RNA]]. Transmission to humans requires mosquito species capable of creating a "bridge" between infected animals and uninfected humans. The [[incubation period]] is 5-15 days.<ref name=CDCSLEVFAQ> Saint Louis Encephalitis. Centers for Disease Control and Prevention (CDC), National Center for Emerging and Zoonotic Infectious Diseases, Division of Vector-Borne Diseases. (2010) http://www.cdc.gov/sle/general/qa.html Accessed on May 3, 2016. </ref> Humans are dead-end hosts for the virus, meaning there is an insufficient amount of St. Louis encephalitis virus in the blood stream to infect a mosquito; there is also no evidence of person to person spread.<ref name=CDCSLEVTransmis> Saint Louis Encephalitis Transmission. Centers for Disease Control and Prevention (CDC), National Center for Emerging and Zoonotic Infectious Diseases, Division of Vector-Borne Diseases. (2010) http://www.cdc.gov/sle/technical/transmission.html Accessed on May 3, 2016. </ref> | ||
St. Louis encephalitis virus | |||
==Pathophysiology== | |||
St. Louis encephalitis virus is usually transmitted via [[mosquito]]s to the human host. St. Louis encephalitis virus contains [[positive-sense ssRNA virus|positive-sense]] viral [[RNA]]; this RNA has its genome directly utilized as if it were mRNA, producing a single protein which is modified by host and viral proteins to form the various proteins needed for [[replication]]. The following table is a summary of the St. Louis encephalitis virus:<ref name=ViralZoneFlavi> Flavivirus. SIB Swiss Institute of Bioinformatics. (2015) http://viralzone.expasy.org/viralzone/all_by_species/24.html Accessed on April 12, 2016</ref><ref name=CDCJapEnceph1> Japanese encephalitis - Frequently Asked Questions. CDC Centers for Disease Control and Prevention. (2015) http://www.cdc.gov/japaneseencephalitis/qa/index.html Accessed on April 12, 2016</ref> | |||
=== | {| class="wikitable" | ||
! style="text-align: center; font-weight: bold;" | Characteristic | |||
! style="text-align: center; font-weight: bold;" | Data | |||
|- | |||
| Nucleic acid | |||
| [[RNA]] | |||
|- | |||
| Sense | |||
| [[positive-sense ssRNA virus|ssRNA(+)]] | |||
|- | |||
| Virion | |||
| [[enveloped virus|Enveloped]] | |||
|- | |||
| [[Capsid]] | |||
| Spherical | |||
|- | |||
| Symmetry | |||
| Yes; T=3-like organization; [[icosahedral]]-like | |||
|- | |||
| Capsid [[monomers]] | |||
| Unknown | |||
|- | |||
| Envelope length (diameter) | |||
| 50 nm | |||
|- | |||
| Additional envelope information | |||
| Mature virons contain 2 virus-encoded membrane proteins (M and E); immature virons contain a protein precursor | |||
|- | |||
| Genome shape | |||
| Linear | |||
|- | |||
| Genome length | |||
| 10-11 kb | |||
|- | |||
| [[Nucleotide]] cap | |||
| Yes | |||
|- | |||
| [[Polyadenylation|Polyadenylated]] tail | |||
| No; a loop structure is formed instead | |||
|- | |||
| [[Incubation period]] | |||
| 5-15 days | |||
|} | |||
St. Louis encephalitis is contracted by the [[bite]] of an infected [[mosquito]], primarily ''Culex pipiens'', ''Culex tarsalis'', or ''Culex nigripalpus''. St. Louis encephalitis virus circulates between a mosquito vector and birds in the United States and South America.<ref name=IDSAEnceph> The Management of Encephalitis: Clinical Practice Guidelines by the Infectious Diseases Society of America. http://www.idsociety.org/uploadedFiles/IDSA/Guidelines-Patient_Care/PDF_Library/Encephalitis.pdf Accessed on May 3, 2016.</ref> Transmission to humans requires mosquito species capable of creating a "bridge" between infected animals and uninfected humans; this occurs when humans become part of the enzootic cycle.<ref name=CDCSLEVFAQ> Saint Louis Encephalitis. Centers for Disease Control and Prevention (CDC), National Center for Emerging and Zoonotic Infectious Diseases, Division of Vector-Borne Diseases. (2010) http://www.cdc.gov/sle/general/qa.html Accessed on May 3, 2016. </ref> Humans are dead-end hosts for the virus, meaning there is an insufficient amount of St. Louis encephalitis virus in the blood stream to infect another human. There is no evidence of St. Louis encephalitis transmission from person to person.<ref name=CDCSLEVTransmis> Saint Louis Encephalitis Transmission. Centers for Disease Control and Prevention (CDC), National Center for Emerging and Zoonotic Infectious Diseases, Division of Vector-Borne Diseases. (2010) http://www.cdc.gov/sle/technical/transmission.html Accessed on May 3, 2016. </ref> | |||
St. Louis encephalitis virus is transmitted in the following pattern:<ref name=ViralZoneFlavi> Flavivirus. SIB Swiss Institute of Bioinformatics. (2015) http://viralzone.expasy.org/viralzone/all_by_species/24.html Accessed on May 3, 2016</ref> | |||
#Attachment of the [[viral envelope]] protein E to host receptors mediates internalization into the host cell by [[clathrin|clathrin-mediated]] [[endocytosis]], or by [[apoptotic]] mimicry. | |||
#Fusion of [[biological membrane|virus membrane]] with host [[endosome|endosomal]] membrane. RNA genome is released into the cytoplasm. | |||
The | #The [[positive-sense ssRNA virus]] is [[translate]]d into a [[polyprotein]], which is cleaved into all structural and non-structural proteins necessary for RNA synthesis ([[replication]] and [[transcription]]). | ||
#[[Replication]] takes place at the surface of [[endoplasmic reticulum]] in [[cytoplasm]]ic viral factories. A [[dsRNA]] [[genome]] is synthesized from the genomic ssRNA(+). | |||
#The [[dsRNA]] [[genome]] is [[transcribed]]/[[replicated]], thereby providing viral mRNAs/new ssRNA(+) genomes. | |||
#Virus assembly occurs at the [[endoplasmic reticulum]]. The virion buds via the host endosomal sorting complexes required for transport (ESCRT), and is sent to the [[Golgi apparatus]]. | |||
#The prM protein is cleaved in the Golgi, thereby maturing the [[virion]] which is fusion competent. | |||
#New virions are released by [[exocytosis]]. | |||
===Histopathological=== | |||
On microscopic histopathological analysis, the enveloped, spherical, and [[icosahedral]]-like virion shape are characteristic findings of St. Louis encephalitis. | |||
===Genetics=== | |||
The [[phylogeny]], [[genetic variation]], and [[Genetic recombination|recombination]] dynamics, and [[Envelope (biology)|envelope protein]] genes St. Louis encephalitis virion have been sequenced.<ref name=KramerPresser>Kramer LD, Presser SB, Hardy JL, Jackson AO. (1997) Genotypic and phenotypic variation of selected Saint Louis encephalitis viral strains isolated in California. ''American Journal of Tropical Medicine and Hygiene'' 57(2):222–229. [http://www.ajtmh.org/cgi/content/abstract/57/2/222 Abstract]</ref><ref name=KramerChandler>Kramer LD, Chandler LJ. (2001) Phylogenetic analysis of the envelope gene of St. Louis encephalitis virus. ''Archives of Virology'' 146(12):2341–2355. {{doi|10.1007/s007050170007}}.</ref><ref name=TwiddyHolmes>Twiddy SS, Holmes EC. (2003) The extent of homologous recombination in members of the genus ''Flavivirus''. ''Journal of General Virology'' 84:429-440. {{doi|10.1099/vir.0.18660-0}}.</ref><ref name=May2008JGV>May FJ, Li L, Zhang S, Guzman H, Beasley DW, Tesh RB, Higgs S, Raj P, Bueno R Jr, Randle Y, Chandler L, Barrett AD. (2008) Genetic variation of St. Louis encephalitis virus. ''Journal of General Virology'' 89(8):1901-1910. {{doi|10.1099/vir.0.2008/000190-0}}.</ref> | |||
Evolutionary evidence suggests that North American strains of St. Louis encephalitis virus belonged to a single [[clade]].<ref name=Baillie>Baillie GJ, Kolokotronis SO, Waltari E, Maffei JG, Kramer LD, Perkins SL. (2008) Phylogenetic and evolutionary analyses of St. Louis encephalitis virus genomes. ''Molecular Phylogenetics and Evolution'' 47(2):717-728. {{doi|10.1016/j.ympev.2008.02.015}}.</ref> Furthermore, this study found an increase in the [[effective population size]] of the St. Louis encephalitis virus around the end of the 19th century that corresponds to the split of the latest North American clade; this finding suggests a northwards colonization of St. Louis encephalitis virus in the Americas. The virus is believed to have originated northern Mexico and migrated northwards with birds. | |||
==Gallery== | ==Gallery== | ||
<gallery> | <gallery> | ||
Image: Flavivirus03.jpeg| Diagram illustrates the methods by which the arbovirus, St. Louis encephalitis virus | Image: CULEXT1.jpg| ''Culex tarsalis'', one of the major vectors of St. Louis encephalitis virus. | ||
Image: Flavivirus01.jpeg| Diagram illustrates the methods by which the arbovirus, St. Louis encephalitis virus | Image: Flavivirus03.jpeg| Diagram illustrates the methods by which the arbovirus, St. Louis encephalitis virus, reproduces and amplifies itself in urban avian populations, and transmitted to dead end hosts including humans and other mammals by ''Culex spp.'' mosquitos. <SMALL><SMALL>''[http://phil.cdc.gov/phil/home.asp From Public Health Image Library (PHIL).] ''<ref name=PHIL> {{Cite web | title = Public Health Image Library (PHIL) | url = http://phil.cdc.gov/phil/home.asp}}</ref></SMALL></SMALL> | ||
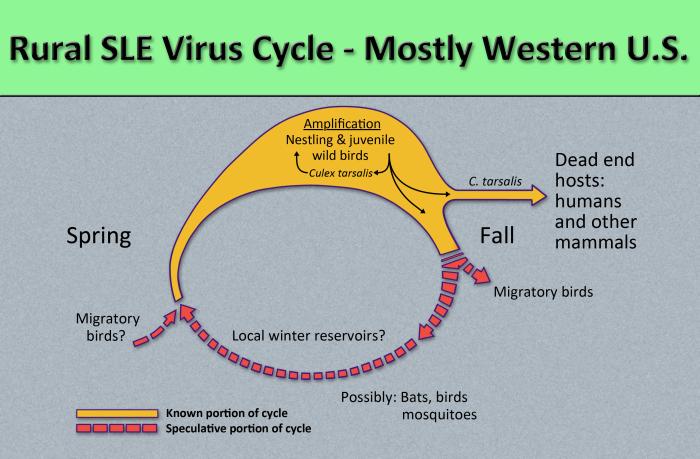
Image: Flavivirus01.jpeg| Diagram illustrates the methods by which the arbovirus, St. Louis encephalitis virus, reproduces and amplifies itself in rural avian populations, and transmitted to dead end hosts including humans and other mammals by the ''Culex tarsalis'' mosquito. <SMALL><SMALL>''[http://phil.cdc.gov/phil/home.asp From Public Health Image Library (PHIL).] ''<ref name=PHIL> {{Cite web | title = Public Health | |||
</gallery> | </gallery> | ||
==References== | ==References== | ||
{{reflist|2}} | {{reflist|2}} | ||
[[Category:Neurology]] | [[Category:Neurology]] | ||
{{ | |||
{{WS}} | |||
{{WH}} | |||
Latest revision as of 19:07, 18 September 2017
|
St. Louis encephalitis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
St. Louis encephalitis pathophysiology On the Web |
|
American Roentgen Ray Society Images of St. Louis encephalitis pathophysiology |
|
Risk calculators and risk factors for St. Louis encephalitis pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Anthony Gallo, B.S. [2]; Contributor(s): Irfan Dotani [3], Vishnu Vardhan Serla M.B.B.S. [4]
Overview
St. Louis encephalitis virus is usually transmitted via mosquitos (generally from the genus Culex) to the human host. St. Louis encephalitis virus contains positive-sense viral RNA. Transmission to humans requires mosquito species capable of creating a "bridge" between infected animals and uninfected humans. The incubation period is 5-15 days.[1] Humans are dead-end hosts for the virus, meaning there is an insufficient amount of St. Louis encephalitis virus in the blood stream to infect a mosquito; there is also no evidence of person to person spread.[2]
Pathophysiology
St. Louis encephalitis virus is usually transmitted via mosquitos to the human host. St. Louis encephalitis virus contains positive-sense viral RNA; this RNA has its genome directly utilized as if it were mRNA, producing a single protein which is modified by host and viral proteins to form the various proteins needed for replication. The following table is a summary of the St. Louis encephalitis virus:[3][4]
| Characteristic | Data |
|---|---|
| Nucleic acid | RNA |
| Sense | ssRNA(+) |
| Virion | Enveloped |
| Capsid | Spherical |
| Symmetry | Yes; T=3-like organization; icosahedral-like |
| Capsid monomers | Unknown |
| Envelope length (diameter) | 50 nm |
| Additional envelope information | Mature virons contain 2 virus-encoded membrane proteins (M and E); immature virons contain a protein precursor |
| Genome shape | Linear |
| Genome length | 10-11 kb |
| Nucleotide cap | Yes |
| Polyadenylated tail | No; a loop structure is formed instead |
| Incubation period | 5-15 days |
St. Louis encephalitis is contracted by the bite of an infected mosquito, primarily Culex pipiens, Culex tarsalis, or Culex nigripalpus. St. Louis encephalitis virus circulates between a mosquito vector and birds in the United States and South America.[5] Transmission to humans requires mosquito species capable of creating a "bridge" between infected animals and uninfected humans; this occurs when humans become part of the enzootic cycle.[1] Humans are dead-end hosts for the virus, meaning there is an insufficient amount of St. Louis encephalitis virus in the blood stream to infect another human. There is no evidence of St. Louis encephalitis transmission from person to person.[2]
St. Louis encephalitis virus is transmitted in the following pattern:[3]
- Attachment of the viral envelope protein E to host receptors mediates internalization into the host cell by clathrin-mediated endocytosis, or by apoptotic mimicry.
- Fusion of virus membrane with host endosomal membrane. RNA genome is released into the cytoplasm.
- The positive-sense ssRNA virus is translated into a polyprotein, which is cleaved into all structural and non-structural proteins necessary for RNA synthesis (replication and transcription).
- Replication takes place at the surface of endoplasmic reticulum in cytoplasmic viral factories. A dsRNA genome is synthesized from the genomic ssRNA(+).
- The dsRNA genome is transcribed/replicated, thereby providing viral mRNAs/new ssRNA(+) genomes.
- Virus assembly occurs at the endoplasmic reticulum. The virion buds via the host endosomal sorting complexes required for transport (ESCRT), and is sent to the Golgi apparatus.
- The prM protein is cleaved in the Golgi, thereby maturing the virion which is fusion competent.
- New virions are released by exocytosis.
Histopathological
On microscopic histopathological analysis, the enveloped, spherical, and icosahedral-like virion shape are characteristic findings of St. Louis encephalitis.
Genetics
The phylogeny, genetic variation, and recombination dynamics, and envelope protein genes St. Louis encephalitis virion have been sequenced.[6][7][8][9]
Evolutionary evidence suggests that North American strains of St. Louis encephalitis virus belonged to a single clade.[10] Furthermore, this study found an increase in the effective population size of the St. Louis encephalitis virus around the end of the 19th century that corresponds to the split of the latest North American clade; this finding suggests a northwards colonization of St. Louis encephalitis virus in the Americas. The virus is believed to have originated northern Mexico and migrated northwards with birds.
Gallery
-
Culex tarsalis, one of the major vectors of St. Louis encephalitis virus.
-
Diagram illustrates the methods by which the arbovirus, St. Louis encephalitis virus, reproduces and amplifies itself in urban avian populations, and transmitted to dead end hosts including humans and other mammals by Culex spp. mosquitos. From Public Health Image Library (PHIL). [11]
-
title = Public Health
References
- ↑ 1.0 1.1 Saint Louis Encephalitis. Centers for Disease Control and Prevention (CDC), National Center for Emerging and Zoonotic Infectious Diseases, Division of Vector-Borne Diseases. (2010) http://www.cdc.gov/sle/general/qa.html Accessed on May 3, 2016.
- ↑ 2.0 2.1 Saint Louis Encephalitis Transmission. Centers for Disease Control and Prevention (CDC), National Center for Emerging and Zoonotic Infectious Diseases, Division of Vector-Borne Diseases. (2010) http://www.cdc.gov/sle/technical/transmission.html Accessed on May 3, 2016.
- ↑ 3.0 3.1 Flavivirus. SIB Swiss Institute of Bioinformatics. (2015) http://viralzone.expasy.org/viralzone/all_by_species/24.html Accessed on April 12, 2016
- ↑ Japanese encephalitis - Frequently Asked Questions. CDC Centers for Disease Control and Prevention. (2015) http://www.cdc.gov/japaneseencephalitis/qa/index.html Accessed on April 12, 2016
- ↑ The Management of Encephalitis: Clinical Practice Guidelines by the Infectious Diseases Society of America. http://www.idsociety.org/uploadedFiles/IDSA/Guidelines-Patient_Care/PDF_Library/Encephalitis.pdf Accessed on May 3, 2016.
- ↑ Kramer LD, Presser SB, Hardy JL, Jackson AO. (1997) Genotypic and phenotypic variation of selected Saint Louis encephalitis viral strains isolated in California. American Journal of Tropical Medicine and Hygiene 57(2):222–229. Abstract
- ↑ Kramer LD, Chandler LJ. (2001) Phylogenetic analysis of the envelope gene of St. Louis encephalitis virus. Archives of Virology 146(12):2341–2355. doi:10.1007/s007050170007.
- ↑ Twiddy SS, Holmes EC. (2003) The extent of homologous recombination in members of the genus Flavivirus. Journal of General Virology 84:429-440. doi:10.1099/vir.0.18660-0.
- ↑ May FJ, Li L, Zhang S, Guzman H, Beasley DW, Tesh RB, Higgs S, Raj P, Bueno R Jr, Randle Y, Chandler L, Barrett AD. (2008) Genetic variation of St. Louis encephalitis virus. Journal of General Virology 89(8):1901-1910. doi:10.1099/vir.0.2008/000190-0.
- ↑ Baillie GJ, Kolokotronis SO, Waltari E, Maffei JG, Kramer LD, Perkins SL. (2008) Phylogenetic and evolutionary analyses of St. Louis encephalitis virus genomes. Molecular Phylogenetics and Evolution 47(2):717-728. doi:10.1016/j.ympev.2008.02.015.
- ↑ "Public Health Image Library (PHIL)".

![Diagram illustrates the methods by which the arbovirus, St. Louis encephalitis virus, reproduces and amplifies itself in urban avian populations, and transmitted to dead end hosts including humans and other mammals by Culex spp. mosquitos. From Public Health Image Library (PHIL). [11]](/images/5/56/Flavivirus03.jpeg)