Silibinin: Difference between revisions
m (Protected "Silibinin": Protecting pages from unwanted edits ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
No edit summary |
||
| Line 1: | Line 1: | ||
{{Drugbox| | {{Drugbox | ||
|IUPAC_name = | | Verifiedfields = changed | ||
| image = Silibinin | | verifiedrevid = 464390273 | ||
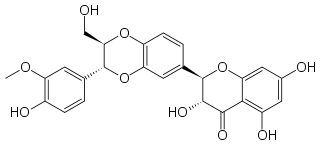
| image2 = | | IUPAC_name = (2''R'',3''R'')-3,5,7-trihydroxy-<br />2-[(2''R'',3''R'')-3-(4-hydroxy-3-methoxyphenyl)-2-(hydroxymethyl)<br />-2,3-dihydrobenzo[''b''][1,4]dioxin-6-yl]chroman-4-one | ||
| CAS_number= 22888-70-6 | | image = Silibinin.png | ||
| ATC_prefix= | | image2 = Silibinin0.png | ||
| ATC_suffix= | |||
| PubChem= 31553 | <!--Clinical data--> | ||
| | | tradename = | ||
| C = 25 |H = 22 |O = 10 | | Drugs.com = {{drugs.com|international|silibinin}} | ||
| routes_of_administration = [[Oral administration|Oral]] [[Intravenous therapy|Intravenous]] | |||
<!--Identifiers--> | |||
| CAS_number_Ref = {{cascite|changed|??}} | |||
| CAS_number = 22888-70-6 | |||
| ATC_prefix = A05 | |||
| ATC_suffix = BA03 | |||
| PubChem = 31553 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 29263 | |||
| ChEBI_Ref = {{ebicite|changed|EBI}} | |||
| ChEBI = 9144 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 4RKY41TBTF | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D08515 | |||
| ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| ChEMBL = 9509 | |||
<!--Chemical data--> | |||
| C=25 | H=22 | O=10 | |||
| molecular_weight = 482.44 g/mol | | molecular_weight = 482.44 g/mol | ||
| | | smiles = O=C4c5c(O)cc(O)cc5O[C@H](c2ccc1O[C@@H]([C@H](Oc1c2)c3ccc(O)c(OC)c3)CO)[C@H]4O | ||
| | | InChI = 1/C25H22O10/c1-32-17-6-11(2-4-14(17)28)24-20(10-26)33-16-5-3-12(7-18(16)34-24)25-23(31)22(30)21-15(29)8-13(27)9-19(21)35-25/h2-9,20,23-29,31H,10H2,1H3/t20-,23+,24-,25-/m1/s1 | ||
| | | InChIKey = SEBFKMXJBCUCAI-HKTJVKLFBV | ||
| | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | | StdInChI = 1S/C25H22O10/c1-32-17-6-11(2-4-14(17)28)24-20(10-26)33-16-5-3-12(7-18(16)34-24)25-23(31)22(30)21-15(29)8-13(27)9-19(21)35-25/h2-9,20,23-29,31H,10H2,1H3/t20-,23+,24-,25-/m1/s1 | ||
| | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | | StdInChIKey = SEBFKMXJBCUCAI-HKTJVKLFSA-N | ||
}} | }} | ||
__NOTOC__ | |||
Chemically modified silibinin, silibinin dihydrogen disuccinate disodium (trade name '''Legalon SIL''') a solution for [[injection (medicine)|injection]], is | {{SI}} | ||
{{CMG}} | |||
==Overview== | |||
'''Silibinin''' ([[International Nonproprietary Name|INN]]), also known as '''silybin '''(both from ''[[Silybum]]'', the [[Genus–differentia definition|generic]] name of the [[plant]] from which it is extracted), is the major active constituent of '''silymarin''', a standardized extract of the [[Silybum marianum|milk thistle]] seeds, containing a mixture of [[flavonolignan]]s consisting of silibinin, [[isosilibinin]], [[silicristin]], [[silidianin]] and others. Silibinin itself is mixture of two [[diastereomer]]s, silybin A and silybin B, in approximately equimolar ratio.<ref>Davis-Searles P, Nakanishi, Y, Nam-Cheol K, et al. (2005). "Milk Thistle and Prostate Cancer: Differential Effects of Pure Flavonolignans from Silybum marianum on Antiproliferative End Points in Human Prostate Carcinoma Cells" ''Cancer Research'' '''65''' (10):4448-57. [http://cancerres.aacrjournals.org/content/65/10/4448.full.pdf doi:10.1158/0008-5472.CAN-04-4662]</ref> Both ''in vitro'' and animal research suggest that silibinin has [[hepatoprotective]] (antihepatotoxic) properties that protect liver cells against toxins.<ref name=AlAnati2009/><ref name=Jayaraj2007/> Silibinin has also demonstrated ''in vitro'' anti-cancer effects against human prostate adenocarcinoma cells, estrogen-dependent and -independent human breast carcinoma cells, human ectocervical carcinoma cells, human colon cancer cells, and both small and nonsmall human lung carcinoma cells.<ref name=Mokhtari2008/><ref name=Bhatia1999/><ref name=Hogan2007/><ref name=Sharma2003/> | |||
Chemically modified silibinin, silibinin dihydrogen disuccinate disodium (trade name '''Legalon SIL'''), a solution for [[injection (medicine)|injection]], is currently being tested as a treatment of severe intoxications with hepatotoxic substances, such as [[Amanita phalloides|death cap]] (''Amanita phalloides'') poisoning.<ref name=Mitchell2009/> There is also clinical evidence for the use of silibinin as a supportive element in alcoholic and child grade 'A' [[liver cirrhosis]].<ref name=Saller2008/> | |||
==Pharmacology== | ==Pharmacology== | ||
Poor water solubility and [[bioavailability]] of silymarin led to the development of enhanced formulations. '''Silipide''' (trade name '''Siliphos'''), a complex of silymarin and [[phosphatidylcholine]] (lecithin), is about | Poor water solubility and [[bioavailability]] of silymarin led to the development of enhanced formulations. '''Silipide''' (trade name '''Siliphos'''), a complex of silymarin and [[phosphatidylcholine]] (lecithin), is about 10 times more bioavailable than silymarin.<ref name=Kidd2005/> An earlier [http://www.phytosomes.info/public/siliphos.asp#references study] had concluded Siliphos to have 4.6 fold higher bioavailability.<ref>Barzaghi N, Crema F, Gatti G, Pifferi G, Perucca E. Eur J Drug Metab Pharmacokinet 1990;15:333–8.</ref> It has been also reported that silymarin inclusion complex with β-[[cyclodextrin]] is much more soluble than silymarin itself.<ref name=Voinovich2009/> There have also been prepared [[glycoside]]s of silybin, which show better water solubility and even stronger hepatoprotective effect.<ref name=Kosina2002/> Silibinin has been reported to exert a neuroprotective effect in mice.<ref>{{cite journal | pmid = 21382422 | year = 2011 | last1 = Tota | first1 = S | last2 = Kamat | first2 = PK | last3 = Shukla | first3 = R | last4 = Nath | first4 = C | title = Improvement of brain energy metabolism and cholinergic functions contributes to the beneficial effects of silibinin against streptozotocin induced memory impairment | volume = 221 | issue = 1 | pages = 207–15 | doi = 10.1016/j.bbr.2011.02.041 | journal = Behavioural Brain Research}}</ref> | ||
Silymarin is the first ingredient in several products sold as herbal telomerase activators, though the research demonstrating silymarin's effectiveness in this regard is proprietary and unpublished. | |||
Silymarin, as other [[flavonoid]]s, has been shown to inhibit P- | Silymarin, as other [[flavonoid]]s, has been shown to inhibit [[P-glycoprotein]]-mediated cellular efflux.<ref name=Zhou2004/> The modulation of P-glycoprotein activity may result in altered absorption and bioavailability of drugs that are P-glycoprotein substrates. It has been reported that silymarin inhibits [[cytochrome P450]] enzymes and an interaction with drugs primarily cleared by P450s cannot be excluded.<ref name=Wu2009/> | ||
==Toxicity== | ==Toxicity== | ||
== | A [[phase I clinical trial]] in humans with prostate cancer designed to study the effects of high dose silibinin found 13 grams daily to be well tolerated in patients with advanced prostate cancer with asymptomatic liver toxicity ([[hyperbilirubinemia]] and elevation of [[alanine aminotransferase]]) being the most commonly seen adverse event.<ref>{{cite journal | journal = Investigational New Drugs | year = 2007 | volume = 25 | issue = 2 | pages = 139–146 | title = A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients | author = Thomas W. Flaig, Daniel L. Gustafson, Lih-Jen Su, Joseph A. Zirrolli, Frances Crighton, Gail S. Harrison, A. Scott Pierson, Rajesh Agarwal, L. Michael Glodé | doi=10.1007/s10637-006-9019-2}}</ref> | ||
The compound is also devoid of embryotoxic potential in animal models.<ref name=Fraschini2002/><ref name=Hahn1968/> | |||
== Potential medical uses == | |||
A recent study suggested that silymarin may help patients with [[type II diabetes]] by assisting in blood sugar control.<ref name=Huseini2006/> | |||
Lab experiments on mice showed that silibinin protects the hepatic cells against the toxin [[alpha-amanitin]] which causes ''[[Amanita phalloides]]'' mushroom poisoning. | |||
== Biotechnology == | |||
Silymarin can be produced in [[callus (cell biology)|callus]] and cells suspensions of ''[[Silybum marianum]]'' and substituted [[pyrazinecarboxamide]]s can be used as abiotic [[elicitor]]s of flavolignan production.<ref>Substituted Pyrazinecarboxamides as Abiotic Elicitors of Flavolignan Production in Silybum marianum (L.) Gaertn Cultures in Vitro. Lenka Tůmová, Jiří Tůma, Klara Megušar, and Martin Doleža, Molecules, 2010, 15(1), pages 331-340, {{doi|10.3390/molecules15010331}}</ref> | |||
== See also == | |||
* [[Sulfad]], a drug containing silibinin. | |||
== References == | |||
{{Reflist|refs= | |||
<ref name=AlAnati2009>{{cite journal |author=Al-Anati L, Essid E, Reinehr R, Petzinger E |title=Silibinin protects OTA-mediated TNF-alpha release from perfused rat livers and isolated rat Kupffer cells |journal=Molecular Nutrition & Food Research |volume=53 |issue=4 |pages=460–6 |year=2009 |pmid=19156713 |doi=10.1002/mnfr.200800110}}</ref> | |||
<ref name=Bhatia1999>{{cite journal |author=Bhatia N, Zhao J, Wolf DM, Agarwal R |title=Inhibition of human carcinoma cell growth and DNA synthesis by silibinin, an active constituent of milk thistle: comparison with silymarin |journal=Cancer Letters |volume=147 |issue=1–2 |pages=77–84 |year=1999 |pmid=10660092 |doi=10.1016/S0304-3835(99)00276-1}}</ref> | |||
<ref name=Hogan2007>{{cite journal |author=Hogan FS, Krishnegowda NK, Mikhailova M, Kahlenberg MS |title=Flavonoid, silibinin, inhibits proliferation and promotes cell-cycle arrest of human colon cancer |journal=Journal of Surgical Research |volume=143 |issue=1 |pages=58–65 |year=2007 |pmid=17950073 |doi=10.1016/j.jss.2007.03.080 |url=http://linkinghub.elsevier.com/retrieve/pii/S0022-4804(07)00241-7}}</ref> | |||
<ref name=Huseini2006>{{cite journal |author=Huseini HF, Larijani B, Heshmat R, Fakhrzadeh H, Radjabipour B, Toliat T, Raza M |title=The efficacy of ''Silybum marianum'' (L.) Gaertn. (silymarin) in the treatment of type II diabetes: a randomized, double-blind, placebo-controlled, clinical trial |journal=Phytotherapy Research |volume=20 |issue=12 |pages=1036–9 |year=2006 |pmid=17072885 |doi=10.1002/ptr.1988}}</ref> | |||
<ref name=Jayaraj2007>{{cite journal |author=Jayaraj R, Deb U, Bhaskar AS, Prasad GB, Rao PV |title=Hepatoprotective efficacy of certain flavonoids against microcystin induced toxicity in mice |journal=Environmental Toxicology |volume=22 |issue=5 |pages=472–9 |year=2007 |pmid=17696131 |doi=10.1002/tox.20283}}</ref> | |||
<ref name=Kidd2005>{{cite journal |author=Kidd P, Head K |title=A review of the bioavailability and clinical efficacy of milk thistle phytosome: a silybin-phosphatidylcholine complex (Siliphos) |journal=Alternative Medicine Review : a Journal of Clinical Therapeutic |volume=10 |issue=3 |pages=193–203 |year=2005 |pmid=16164374 |doi= |url=http://www.thorne.com/altmedrev/.fulltext/10/3/193.pdf |accessdate=2010-12-14}}</ref> | |||
<ref name=Kosina2002>{{cite journal |author=Kosina P, Kren V, Gebhardt R, Grambal F, Ulrichová J, Walterová D |title=Antioxidant properties of silybin glycosides |journal=Phytotherapy Research : PTR |volume=16 Suppl 1 |issue= |pages=S33–9 |year=2002 |pmid=11933137 |doi= 10.1002/ptr.796}}</ref> | |||
<ref name=Mitchell2009>{{cite journal |author=Mitchell, T |title=Intravenous Milk thistle (silibinin-legalon) for hepatic failure induced by amatoxin/''Amanita'' mushroom poisoning |journal=(Clinical study) |year=2009 |url=http://clinicaltrials.gov/ct2/show/NCT00915681}}</ref> | |||
<ref name=Mokhtari2008>{{cite journal |author=Mokhtari MJ, Motamed N, Shokrgozar MA |title=Evaluation of silibinin on the viability, migration and adhesion of the human prostate adenocarcinoma (PC-3) cell line |journal=Cell Biology International |volume=32 |issue=8 |pages=888–92 |year=2008 |pmid=18538589 |doi=10.1016/j.cellbi.2008.03.019 |url=http://linkinghub.elsevier.com/retrieve/pii/S1065-6995(08)00384-3}}</ref> | |||
<ref name=Saller2008>{{cite journal |author=Saller R, Brignoli R, Melzer J, Meier R |title=An updated systematic review with meta-analysis for the clinical evidence of silymarin |journal=Forschende Komplementärmedizin (2006) |volume=15 |issue=1 |pages=9–20 |year=2008 |pmid=18334810 |doi=10.1159/000113648 |url=http://content.karger.com/produktedb/produkte.asp?typ=fulltext&file=000113648 |accessdate=2010-12-14}}</ref> | |||
<ref name=Sharma2003>{{cite journal |author=Sharma G, Singh RP, Chan DC, Agarwal R |title=Silibinin induces growth inhibition and apoptotic cell death in human lung carcinoma cells |journal=Anticancer Research |volume=23 |issue=3B |pages=2649–55 |year=2003 |pmid=12894553}}</ref> | |||
<ref name=Voinovich2009>{{cite journal |author=Voinovich D, Perissutti B, Grassi M, Passerini N, Bigotto A |title=Solid state mechanochemical activation of ''Silybum marianum'' dry extract with betacyclodextrins: Characterization and bioavailability of the coground systems |journal=Journal of Pharmaceutical Sciences |volume=98 |issue=11 |pages=4119–29 |year=2009 |pmid=19226635 |doi=10.1002/jps.21704}}</ref> | |||
<ref name=Wu2009>{{cite journal |author=Wu JW, Lin LC, Tsai TH |title=Drug-drug interactions of silymarin on the perspective of pharmacokinetics |journal=Journal of Ethnopharmacology |volume=121 |issue=2 |pages=185–93 |year=2009 |pmid=19041708 |doi=10.1016/j.jep.2008.10.036 |url=http://linkinghub.elsevier.com/retrieve/pii/S0378-8741(08)00621-1 |accessdate=2010-12-14}}</ref> | |||
<ref name=Zhou2004>{{cite journal |author=Zhou S, Lim LY, Chowbay B |title=Herbal modulation of P-glycoprotein |journal=Drug Metabolism Reviews |volume=36 |issue=1 |pages=57–104 |year=2004 |pmid=15072439 |doi=10.1081/DMR-120028427 |url=http://www.informapharmascience.com/doi/abs/10.1081/DMR-120028427 }}</ref> | |||
<ref name=Fraschini2002>{{cite journal |author=Fraschini F, Demartini G, Esposti D |title=Pharmacology of Silymarin |journal=Clinical Drug Investigation |volume=22 |issue=1 |pages=51–65 |year=2002 |doi=10.2165/00044011-200222010-00007 | url=http://adisonline.com/druginvestigation/Abstract/2002/22010/Pharmacology_of_Silymarin.7.aspx }}</ref> | |||
<ref name=Hahn1968>{{cite journal |author=Hahn G, Lehmann HD, Kürten M, Uebel H, Vogel G |title=On the pharmacology and toxicology of silymarin, an antihepatotoxic active principle from Silybum marianum (L.) gaertn |journal=Arzneimittelforschung |volume=18 |issue=6 |pages=698–704 |year=1968 |pmid=5755807 }}</ref> | |||
}} | |||
==References== | ==References== | ||
{{reflist|2}} | |||
{{Bile and liver therapy}} | |||
{{lignan}} | |||
[[Category:Antidotes]] | [[Category:Antidotes]] | ||
[[Category: | [[Category:Flavonolignans]] | ||
[[Category: | [[Category:Phenols]] | ||
[[Category:Drug]] | |||
[[ | |||
Revision as of 15:59, 9 April 2015
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral Intravenous |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C25H22O10 |
| Molar mass | 482.44 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
|
WikiDoc Resources for Silibinin |
|
Articles |
|---|
|
Most recent articles on Silibinin |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Silibinin at Clinical Trials.gov Clinical Trials on Silibinin at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Silibinin
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Silibinin Discussion groups on Silibinin Directions to Hospitals Treating Silibinin Risk calculators and risk factors for Silibinin
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Silibinin |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Silibinin (INN), also known as silybin (both from Silybum, the generic name of the plant from which it is extracted), is the major active constituent of silymarin, a standardized extract of the milk thistle seeds, containing a mixture of flavonolignans consisting of silibinin, isosilibinin, silicristin, silidianin and others. Silibinin itself is mixture of two diastereomers, silybin A and silybin B, in approximately equimolar ratio.[1] Both in vitro and animal research suggest that silibinin has hepatoprotective (antihepatotoxic) properties that protect liver cells against toxins.[2][3] Silibinin has also demonstrated in vitro anti-cancer effects against human prostate adenocarcinoma cells, estrogen-dependent and -independent human breast carcinoma cells, human ectocervical carcinoma cells, human colon cancer cells, and both small and nonsmall human lung carcinoma cells.[4][5][6][7]
Chemically modified silibinin, silibinin dihydrogen disuccinate disodium (trade name Legalon SIL), a solution for injection, is currently being tested as a treatment of severe intoxications with hepatotoxic substances, such as death cap (Amanita phalloides) poisoning.[8] There is also clinical evidence for the use of silibinin as a supportive element in alcoholic and child grade 'A' liver cirrhosis.[9]
Pharmacology
Poor water solubility and bioavailability of silymarin led to the development of enhanced formulations. Silipide (trade name Siliphos), a complex of silymarin and phosphatidylcholine (lecithin), is about 10 times more bioavailable than silymarin.[10] An earlier study had concluded Siliphos to have 4.6 fold higher bioavailability.[11] It has been also reported that silymarin inclusion complex with β-cyclodextrin is much more soluble than silymarin itself.[12] There have also been prepared glycosides of silybin, which show better water solubility and even stronger hepatoprotective effect.[13] Silibinin has been reported to exert a neuroprotective effect in mice.[14]
Silymarin is the first ingredient in several products sold as herbal telomerase activators, though the research demonstrating silymarin's effectiveness in this regard is proprietary and unpublished.
Silymarin, as other flavonoids, has been shown to inhibit P-glycoprotein-mediated cellular efflux.[15] The modulation of P-glycoprotein activity may result in altered absorption and bioavailability of drugs that are P-glycoprotein substrates. It has been reported that silymarin inhibits cytochrome P450 enzymes and an interaction with drugs primarily cleared by P450s cannot be excluded.[16]
Toxicity
A phase I clinical trial in humans with prostate cancer designed to study the effects of high dose silibinin found 13 grams daily to be well tolerated in patients with advanced prostate cancer with asymptomatic liver toxicity (hyperbilirubinemia and elevation of alanine aminotransferase) being the most commonly seen adverse event.[17]
The compound is also devoid of embryotoxic potential in animal models.[18][19]
Potential medical uses
A recent study suggested that silymarin may help patients with type II diabetes by assisting in blood sugar control.[20]
Lab experiments on mice showed that silibinin protects the hepatic cells against the toxin alpha-amanitin which causes Amanita phalloides mushroom poisoning.
Biotechnology
Silymarin can be produced in callus and cells suspensions of Silybum marianum and substituted pyrazinecarboxamides can be used as abiotic elicitors of flavolignan production.[21]
See also
- Sulfad, a drug containing silibinin.
References
- ↑ Davis-Searles P, Nakanishi, Y, Nam-Cheol K, et al. (2005). "Milk Thistle and Prostate Cancer: Differential Effects of Pure Flavonolignans from Silybum marianum on Antiproliferative End Points in Human Prostate Carcinoma Cells" Cancer Research 65 (10):4448-57. doi:10.1158/0008-5472.CAN-04-4662
- ↑ Al-Anati L, Essid E, Reinehr R, Petzinger E (2009). "Silibinin protects OTA-mediated TNF-alpha release from perfused rat livers and isolated rat Kupffer cells". Molecular Nutrition & Food Research. 53 (4): 460–6. doi:10.1002/mnfr.200800110. PMID 19156713.
- ↑ Jayaraj R, Deb U, Bhaskar AS, Prasad GB, Rao PV (2007). "Hepatoprotective efficacy of certain flavonoids against microcystin induced toxicity in mice". Environmental Toxicology. 22 (5): 472–9. doi:10.1002/tox.20283. PMID 17696131.
- ↑ Mokhtari MJ, Motamed N, Shokrgozar MA (2008). "Evaluation of silibinin on the viability, migration and adhesion of the human prostate adenocarcinoma (PC-3) cell line". Cell Biology International. 32 (8): 888–92. doi:10.1016/j.cellbi.2008.03.019. PMID 18538589.
- ↑ Bhatia N, Zhao J, Wolf DM, Agarwal R (1999). "Inhibition of human carcinoma cell growth and DNA synthesis by silibinin, an active constituent of milk thistle: comparison with silymarin". Cancer Letters. 147 (1–2): 77–84. doi:10.1016/S0304-3835(99)00276-1. PMID 10660092.
- ↑ Hogan FS, Krishnegowda NK, Mikhailova M, Kahlenberg MS (2007). "Flavonoid, silibinin, inhibits proliferation and promotes cell-cycle arrest of human colon cancer". Journal of Surgical Research. 143 (1): 58–65. doi:10.1016/j.jss.2007.03.080. PMID 17950073.
- ↑ Sharma G, Singh RP, Chan DC, Agarwal R (2003). "Silibinin induces growth inhibition and apoptotic cell death in human lung carcinoma cells". Anticancer Research. 23 (3B): 2649–55. PMID 12894553.
- ↑ Mitchell, T (2009). "Intravenous Milk thistle (silibinin-legalon) for hepatic failure induced by amatoxin/Amanita mushroom poisoning". (Clinical study).
- ↑ Saller R, Brignoli R, Melzer J, Meier R (2008). "An updated systematic review with meta-analysis for the clinical evidence of silymarin". Forschende Komplementärmedizin (2006). 15 (1): 9–20. doi:10.1159/000113648. PMID 18334810. Retrieved 2010-12-14.
- ↑ Kidd P, Head K (2005). "A review of the bioavailability and clinical efficacy of milk thistle phytosome: a silybin-phosphatidylcholine complex (Siliphos)" (PDF). Alternative Medicine Review : a Journal of Clinical Therapeutic. 10 (3): 193–203. PMID 16164374. Retrieved 2010-12-14.
- ↑ Barzaghi N, Crema F, Gatti G, Pifferi G, Perucca E. Eur J Drug Metab Pharmacokinet 1990;15:333–8.
- ↑ Voinovich D, Perissutti B, Grassi M, Passerini N, Bigotto A (2009). "Solid state mechanochemical activation of Silybum marianum dry extract with betacyclodextrins: Characterization and bioavailability of the coground systems". Journal of Pharmaceutical Sciences. 98 (11): 4119–29. doi:10.1002/jps.21704. PMID 19226635.
- ↑ Kosina P, Kren V, Gebhardt R, Grambal F, Ulrichová J, Walterová D (2002). "Antioxidant properties of silybin glycosides". Phytotherapy Research : PTR. 16 Suppl 1: S33–9. doi:10.1002/ptr.796. PMID 11933137.
- ↑ Tota, S; Kamat, PK; Shukla, R; Nath, C (2011). "Improvement of brain energy metabolism and cholinergic functions contributes to the beneficial effects of silibinin against streptozotocin induced memory impairment". Behavioural Brain Research. 221 (1): 207–15. doi:10.1016/j.bbr.2011.02.041. PMID 21382422.
- ↑ Zhou S, Lim LY, Chowbay B (2004). "Herbal modulation of P-glycoprotein". Drug Metabolism Reviews. 36 (1): 57–104. doi:10.1081/DMR-120028427. PMID 15072439.
- ↑ Wu JW, Lin LC, Tsai TH (2009). "Drug-drug interactions of silymarin on the perspective of pharmacokinetics". Journal of Ethnopharmacology. 121 (2): 185–93. doi:10.1016/j.jep.2008.10.036. PMID 19041708. Retrieved 2010-12-14.
- ↑ Thomas W. Flaig, Daniel L. Gustafson, Lih-Jen Su, Joseph A. Zirrolli, Frances Crighton, Gail S. Harrison, A. Scott Pierson, Rajesh Agarwal, L. Michael Glodé (2007). "A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients". Investigational New Drugs. 25 (2): 139–146. doi:10.1007/s10637-006-9019-2.
- ↑ Fraschini F, Demartini G, Esposti D (2002). "Pharmacology of Silymarin". Clinical Drug Investigation. 22 (1): 51–65. doi:10.2165/00044011-200222010-00007.
- ↑ Hahn G, Lehmann HD, Kürten M, Uebel H, Vogel G (1968). "On the pharmacology and toxicology of silymarin, an antihepatotoxic active principle from Silybum marianum (L.) gaertn". Arzneimittelforschung. 18 (6): 698–704. PMID 5755807.
- ↑ Huseini HF, Larijani B, Heshmat R, Fakhrzadeh H, Radjabipour B, Toliat T, Raza M (2006). "The efficacy of Silybum marianum (L.) Gaertn. (silymarin) in the treatment of type II diabetes: a randomized, double-blind, placebo-controlled, clinical trial". Phytotherapy Research. 20 (12): 1036–9. doi:10.1002/ptr.1988. PMID 17072885.
- ↑ Substituted Pyrazinecarboxamides as Abiotic Elicitors of Flavolignan Production in Silybum marianum (L.) Gaertn Cultures in Vitro. Lenka Tůmová, Jiří Tůma, Klara Megušar, and Martin Doleža, Molecules, 2010, 15(1), pages 331-340, doi:10.3390/molecules15010331
References
- Pages with script errors
- CS1 maint: Multiple names: authors list
- Template:drugs.com link with non-standard subpage
- Articles with changed CASNo identifier
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Chemical pages without DrugBank identifier
- Drugs with no legal status
- Drugboxes which contain changes to verified fields
- Antidotes
- Flavonolignans
- Phenols
- Drug