Sandbox Enoxaparin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: SPINAL/EPIDURAL HEMATOMA
See full prescribing information for complete Boxed Warning.
Epidural or spinal hematomas may occur in patients who are anticoagulated with low molecular weight heparins (LMWH) or heparinoids and are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include:
|
Overview
Sandbox Enoxaparin is {{{aOrAn}}} Low molecular weight heparin that is FDA approved for the {{{indicationType}}} of Treatment of acute deep vein thrombosis,acute ST-segment elevation myocardial infarction and prophylaxis of deep vein thrombosis, ischemic complications of unstable angina and Non-Q-Wave myocardial infarction. There is a Black Box Warning for this drug as shown here. Common adverse reactions include Diarrhea, Nausea,Anemia, Bleeding, Major , Thrombocytopenia,Increased liver function test Fever.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
<h4>Condition 1</h4>
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Sandbox Enoxaparin FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Contraindications
- Active major bleeding
- Thrombocytopenia associated with a positive in vitro test for anti-platelet antibody in the presence of enoxaparin sodium
- Known hypersensitivity to enoxaparin sodium (e.g., pruritus, urticaria, anaphylactic/anaphylactoid reactions) [see Adverse Reactions (6.2)]
- Known hypersensitivity to heparin or pork products
- Known hypersensitivity to benzyl alcohol (which is in only the multi-dose formulation of Lovenox)
Warnings
|
WARNING: SPINAL/EPIDURAL HEMATOMA
See full prescribing information for complete Boxed Warning.
Epidural or spinal hematomas may occur in patients who are anticoagulated with low molecular weight heparins (LMWH) or heparinoids and are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include:
|
Cases of epidural or spinal hemorrhage and subsequent hematomas have been reported with the use of Lovenox and epidural or spinal anesthesia/analgesia or spinal puncture procedures, resulting in long-term or permanent paralysis. The risk of these events is higher with the use of post-operative indwelling epidural catheters, with the concomitant use of additional drugs affecting hemostasis such as NSAIDs, with traumatic or repeated epidural or spinal puncture, or in patients with a history of spinal surgery or spinal deformity [see Boxed Warning, Adverse Reactions (6.2) and Drug Interactions (7)].
Adverse Reactions
Clinical Trials Experience
Clinical Trials Experience
The following serious adverse reactions are also discussed in other sections of the labeling:
- *Spinal/epidural hematoma [see Boxed Warning and Warnings and Precautions (5.1)]
- *Increased Risk of Hemorrhage [see Warnings and Precautions (5.1)]
- *Thrombocytopenia [see Warnings and Precautions (5.5)]
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
During clinical development for the approved indications, 15,918 patients were exposed to enoxaparin sodium. These included 1,228 for prophylaxis of deep vein thrombosisfollowing abdominal surgery in patients at risk for thromboembolic complications, 1,368 for prophylaxis of deep vein thrombosisfollowing hip or knee replacement surgery, 711 for prophylaxis of deep vein thrombosisin medical patients with severely restricted mobility during acute illness, 1,578 for prophylaxis of ischemic complications in unstable angina and non-Q-wave myocardial infarction, 10,176 for treatment of acute ST-elevation myocardial infarction, and 857 for treatment of deep vein thrombosiswith or without pulmonary embolism. Enoxaparin sodium doses in the clinical trials for prophylaxis of deep vein thrombosisfollowing abdominal or hip or knee replacement surgery or in medical patients with severely restricted mobility during acute illness ranged from 40 mg SC once daily to 30 mg SC twice daily. In the clinical studies for prophylaxis of ischemic complications of unstable angina and non-Q-wave myocardial infarction doses were 1 mg/kg every 12 hours and in the clinical studies for treatment of acute ST-segment elevation myocardial infarction enoxaparin sodium doses were a 30 mg IV bolus followed by 1 mg/kg every 12 hours SC.
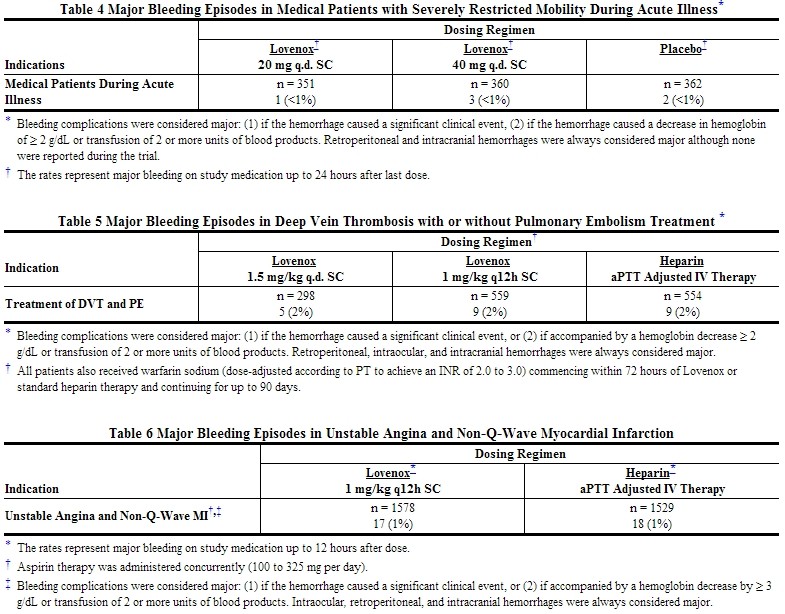
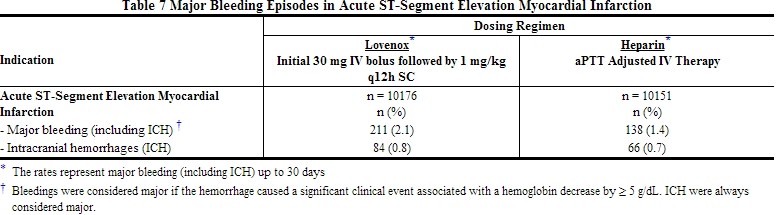
Hemorrhage
The incidence of major hemorrhagic complications during Lovenox treatment has been low.
The following rates of major bleeding events have been reported during clinical trials with Lovenox [see Tables 2 to 7].
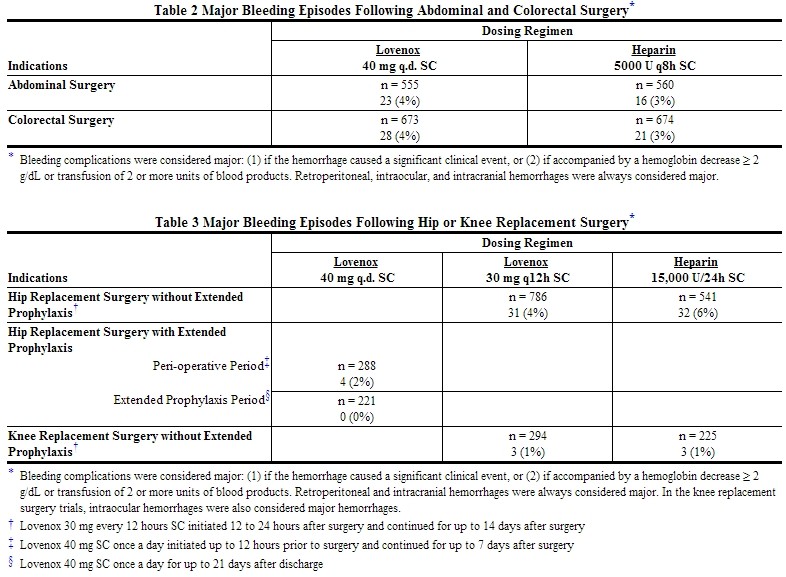
NOTE: At no time point were the 40 mg once a day pre-operative and the 30 mg every 12 hours post-operative hip replacement surgery prophylactic regimens compared in clinical trials.
Injection site hematomas during the extended prophylaxis period after hip replacement surgery occurred in 9% of the Lovenox patients versus 1.8% of the placebo patients.
Elevations of Serum Aminotransferases
Asymptomatic increases in aspartate (AST [SGOT]) and alanine (ALT [SGPT]) aminotransferase levels greater than three times the upper limit of normal of the laboratory reference range have been reported in up to 6.1% and 5.9% of patients, respectively, during treatment with Lovenox. Similar significant increases in aminotransferase levels have also been observed in patients and healthy volunteers treated with heparin and other low molecular weight heparins. Such elevations are fully reversible and are rarely associated with increases in bilirubin.
Since aminotransferase determinations are important in the differential diagnosis of myocardial infarction, liver disease, and pulmonary emboli, elevations that might be caused by drugs like Lovenox should be interpreted with caution.
Local Reactions
Mild local irritation, pain, hematoma, ecchymosis, and erythema may follow SC injection of Lovenox.
Adverse Reactions in Patients Receiving Lovenox for Prophylaxis or Treatment of DVT, PE
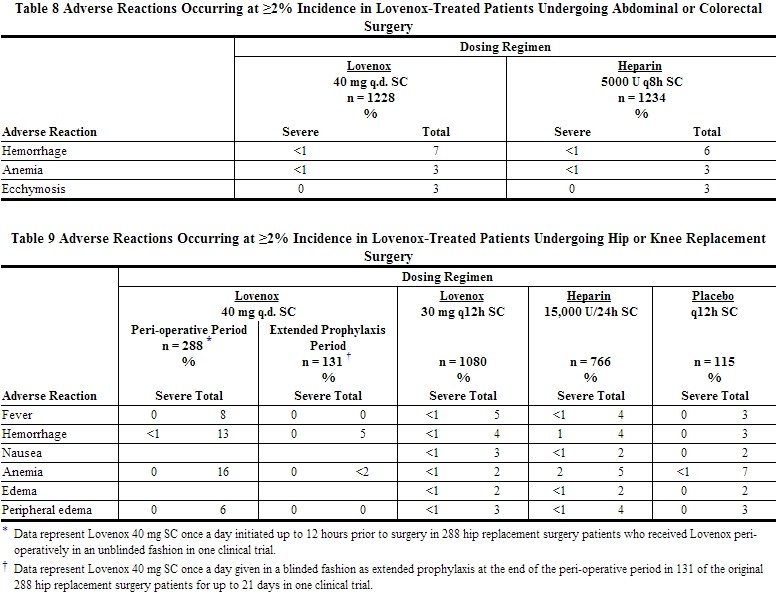
Other adverse reactions that were thought to be possibly or probably related to treatment with Lovenox, heparin, or placebo in clinical trials with patients undergoing hip or knee replacement surgery, abdominal or colorectal surgery, or treatment for DVT and that occurred at a rate of at least 2% in the Lovenox group, are provided below [see Tables 8 to 11].
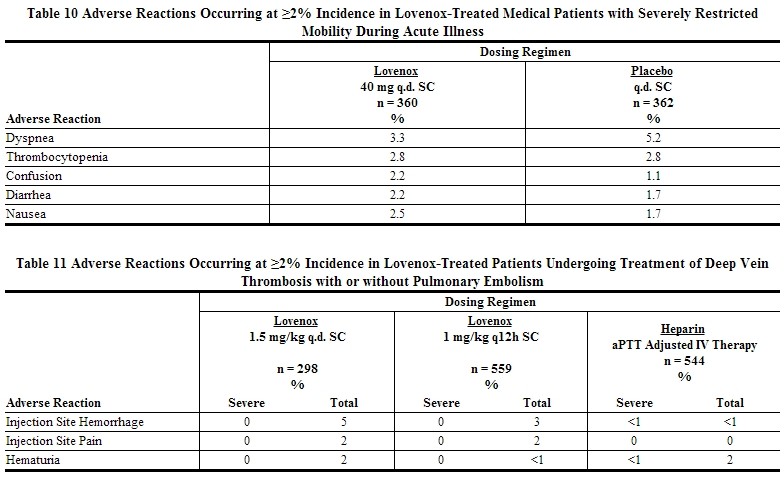
Adverse Events in Lovenox-Treated Patients with unstable angina or Non-Q-Wave myocardial infarction
Non-hemorrhagic clinical events reported to be related to Lovenox therapy occurred at an incidence of ≤1%.
Non-major hemorrhagic events, primarily injection site ecchymoses and hematomas, were more frequently reported in patients treated with SC Lovenox than in patients treated with IV heparin.
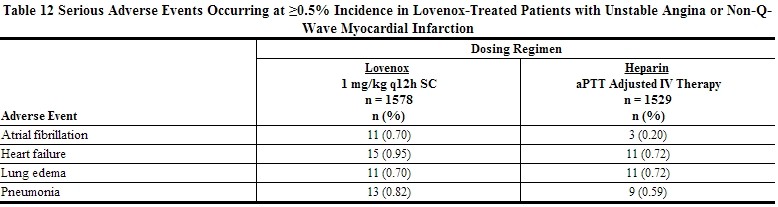
Serious adverse events with Lovenox or heparin in a clinical trial in patients with unstable angina or non-Q-wave myocardial infarction that occurred at a rate of at least 0.5% in the Lovenox group are provided below [see Table 12].
Adverse Reactions in Lovenox-Treated Patients with Acute ST-Segment Elevation myocardial infarction
In a clinical trial in patients with acute ST-segment elevation myocardial infarction, the only adverse reaction that occurred at a rate of at least 0.5% in the Lovenox group was thrombocytopenia (1.5%).
Postmarketing Experience
The following adverse reactions have been identified during postapproval use of Lovenox. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
There have been reports of epidural or spinal hematoma formation with concurrent use of Lovenox and spinal/epidural anesthesia or spinal puncture. The majority of patients had a post-operative indwelling epidural catheter placed for analgesia or received additional drugs affecting hemostasis such as NSAIDs. Many of the epidural or spinal hematomas caused neurologic injury, including long-term or permanent paralysis.
Local reactions at the injection site (e.g. nodules, inflammation, oozing), systemic allergic reactions (e.g. pruritus, urticaria, anaphylactic/anaphylactoid reactions including shock), vesiculobullous rash, rare cases of hypersensitivity cutaneous vasculitis, purpura, skin necrosis (occurring at either the injection site or distant from the injection site), thrombocytosis, and thrombocytopenia with thrombosis [see Warnings and Precautions (5.5)] have been reported.
Cases of hyperkalemia have been reported. Most of these reports occurred in patients who also had conditions that tend toward the development of hyperkalemia (e.g., renal dysfunction, concomitant potassium-sparing drugs, administration of potassium, hematoma in body tissues). Very rare cases of hyperlipidemia have also been reported, with one case of hyperlipidemia, with marked hypertriglyceridemia, reported in a diabetic pregnant woman; causality has not been determined.
Cases of headache, hemorrhagic anemia, eosinophilia, alopecia, hepatocellular and cholestatic liver injury have been reported.
Osteoporosis has also been reported following long-term therapy.
Drug Interactions
Whenever possible, agents which may enhance the risk of hemorrhage should be discontinued prior to initiation of Lovenox therapy. These agents include medications such as: anticoagulants, platelet inhibitors including acetylsalicylic acid, salicylates, NSAIDs (including ketorolac tromethamine), dipyridamole, or sulfinpyrazone. If co-administration is essential, conduct close clinical and laboratory monitoring
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
All pregnancies have a background risk of birth defect, loss, or other adverse outcome regardless of drug exposure. The fetal risk summary below describes the potential of Lovenox to increase the risk of developmental abnormalities above the background risk.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Sandbox Enoxaparin in women who are pregnant.
Labor and Delivery
Lovenox does not cross the placenta, and is not expected to result in fetal exposure to the drug. Human data from a retrospective cohort study, which included 693 live births, suggest that Lovenox does not increase the risk of major developmental abnormalities. Based on animal data, enoxaparin is not predicted to increase the risk of major developmental abnormalities
Nursing Mothers
It is not known whether Lovenox is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from Lovenox, a decision should be made whether to discontinue nursing or discontinue Lovenox, taking into account the importance of Lovenox to the mother and the known benefits of nursing.
Pediatric Use
Safety and effectiveness of Lovenox in pediatric patients have not been established.
Geriatic Use
Prevention of Deep Vein Thrombosis in Hip, Knee and Abdominal Surgery; Treatment of Deep Vein Thrombosis, Prevention of Ischemic Complications of Unstable Angina and Non-Q-wave Myocardial Infarction
Over 2800 patients, 65 years and older, have received Lovenox in pivotal clinical trials. The efficacy of Lovenox in the geriatric (≥65 years) was similar to that seen in younger patients (<65 years). The incidence of bleeding complications was similar between geriatric and younger patients when 30 mg every 12 hours or 40 mg once a day doses of Lovenox were employed. The incidence of bleeding complications was higher in geriatric patients as compared to younger patients when Lovenox was administered at doses of 1.5 mg/kg once a day or 1 mg/kg every 12 hours. The risk of Lovenox-associated bleeding increased with age. Serious adverse events increased with age for patients receiving Lovenox. Other clinical experience (including postmarketing surveillance and literature reports) has not revealed additional differences in the safety of Lovenox between geriatric and younger patients. Careful attention to dosing intervals and concomitant medications (especially antiplatelet medications) is advised. Lovenox should be used with care in geriatric patients who may show delayed elimination of enoxaparin. Monitoring of geriatric patients with low body weight (<45 kg) and those predisposed to decreased renal function should be considered [see Warnings and Precautions (5.9) and Clinical Pharmacology (12.3)].
Treatment of Acute ST-Segment Elevation Myocardial Infarction
In the clinical study for treatment of acute ST-segment elevation myocardial infarction, there was no evidence of difference in efficacy between patients ≥75 years of age (n = 1241) and patients less than 75 years of age (n=9015). Patients ≥75 years of age did not receive a 30 mg IV bolus prior to the normal dosage regimen and had their SC dose adjusted to 0.75 mg/kg every 12 hours [see Dosage and Administration (2.3)]. The incidence of bleeding complications was higher in patients ≥65 years of age as compared to younger patients (<65 years).
Gender
There is no FDA guidance on the use of Sandbox Enoxaparin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Sandbox Enoxaparin with respect to specific racial populations.
Renal Impairment
In patients with renal impairment, there is an increase in exposure of enoxaparin sodium. All such patients should be observed carefully for signs and symptoms of bleeding. Because exposure of enoxaparin sodium is significantly increased in patients with severe renal impairment (creatinine clearance <30 mL/min), a dosage adjustment is recommended for therapeutic and prophylactic dosage ranges. No dosage adjustment is recommended in patients with moderate (creatinine clearance 30–50 mL/min) and mild (creatinine clearance 50–80 mL/min) renal impairment [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)]. In patients with renal failure, treatment with enoxaparin has been associated with the development of hyperkalemia [see Adverse Reactions (6.2)].
Hepatic Impairment
The impact of hepatic impairment on enoxaparin's exposure and antithrombotic effect has not been investigated. Caution should be exercised when administering enoxaparin to patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Sandbox Enoxaparin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Sandbox Enoxaparin in patients who are immunocompromised.
Low-Weight Patients
An increase in exposure of enoxaparin sodium with prophylactic dosages (non-weight adjusted) has been observed in low-weight women (<45 kg) and low-weight men (<57 kg). All such patients should be observed carefully for signs and symptoms of bleeding [see Clinical Pharmacology (12.3)].
Administration and Monitoring
Administration
Lovenox is a clear, colorless to pale yellow sterile solution, and as with other parenteral drug products, should be inspected visually for particulate matter and discoloration prior to administration.
The use of a tuberculin syringe or equivalent is recommended when using Lovenox multiple-dose vials to assure withdrawal of the appropriate volume of drug.
Lovenox must not be administered by intramuscular injection. Lovenox is intended for use under the guidance of a physician.
For subcutaneous administration, patients may self-inject only if their physicians determine that it is appropriate and with medical follow-up, as necessary. Proper training in subcutaneous injection technique (with or without the assistance of an injection device) should be provided.
Subcutaneous Injection Technique: Patients should be lying down and Lovenox administered by deep SC injection. To avoid the loss of drug when using the 30 and 40 mg prefilled syringes, do not expel the air bubble from the syringe before the injection. Administration should be alternated between the left and right anterolateral and left and right posterolateral abdominal wall. The whole length of the needle should be introduced into a skin fold held between the thumb and forefinger; the skin fold should be held throughout the injection. To minimize bruising, do not rub the injection site after completion of the injection.
Lovenox prefilled syringes and graduated prefilled syringes are for single, one-time use only and are available with a system that shields the needle after injection.
Remove the prefilled syringe from the blister packaging by peeling at the arrow as directed on the blister. Do not remove by pulling on the plunger as this may damage the syringe.
1.Remove the needle shield by pulling it straight off the syringe (see Figure A). If adjusting the dose is required, the dose adjustment must be done prior to injecting the prescribed dose to the patient.
Figure A
{
Monitoring
- All patients should be evaluated for a bleeding disorder before administration of Enoxaparin Sodium Injection, unless the medication is needed urgently. Since coagulation parameters are unsuitable for monitoring Enoxaparin Sodium Injection activity, routine monitoring of coagulation parameters is not required
- Thrombocytopenia of any degree should be monitored closely. If the platelet count falls below 100,000/mm3, enoxaparin sodium injection should be discontinued. Cases of heparin-induced thrombocytopenia with thrombosis have also been observed in clinical practice. Some of these cases were complicated by organ infarction, limb ischemia, or death
- Women with mechanical prosthetic heart valves may be at higher risk for thromboembolism during pregnancy, and, when pregnant, have a higher rate of fetal loss from stillbirth, spontaneous abortion and premature delivery. Therefore, frequent monitoring of peak and trough anti-Factor Xa levels, and adjusting of dosage may be needed
- Anti-Factor Xa may be used to monitor the anticoagulant effect of enoxaparin sodium injection in patients with significant renal impairment. If during enoxaparin sodium injection therapy abnormal coagulation parameters or bleeding should occur, anti-Factor Xa levels may be used to monitor the anticoagulant effects of enoxaparin sodium injection
- Whenever possible, agents which may enhance the risk of hemorrhage should be discontinued prior to initiation of enoxaparin sodium injection therapy. These agents include medications such as: anticoagulants, platelet inhibitors including acetylsalicylic acid, salicylates, NSAIDs (including ketorolac tromethamine), dipyridamole, or sulfinpyrazone. If co-administration is essential, conduct close clinical and laboratory monitoring
- Monitoring of geriatric patients with low body weight (<45 kg) and those predisposed to decreased renal function should be considered
IV Compatibility
There is limited information regarding the compatibility of Sandbox Enoxaparin and IV administrations.
Overdosage
There is limited information regarding Sandbox Enoxaparin overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Sandbox Enoxaparin Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Sandbox Enoxaparin Mechanism of Action in the drug label.
Structure
There is limited information regarding Sandbox Enoxaparin Structure in the drug label.
Pharmacodynamics
There is limited information regarding Sandbox Enoxaparin Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Sandbox Enoxaparin Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Sandbox Enoxaparin Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Sandbox Enoxaparin Clinical Studies in the drug label.
How Supplied
There is limited information regarding Sandbox Enoxaparin How Supplied in the drug label.
Storage
There is limited information regarding Sandbox Enoxaparin Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Sandbox Enoxaparin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Sandbox Enoxaparin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Sandbox Enoxaparin Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Sandbox Enoxaparin interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Sandbox Enoxaparin Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Sandbox Enoxaparin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.