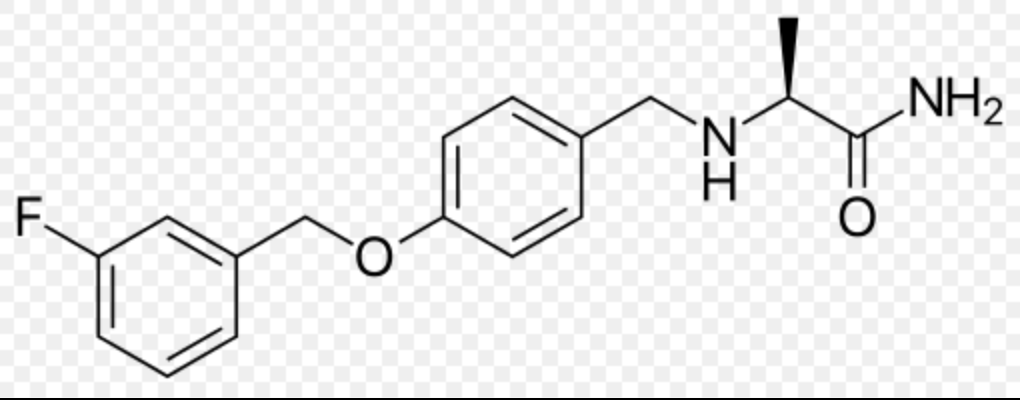
Safinamide
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Yashasvi Aryaputra[2];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Warning Title
See full prescribing information for complete Boxed Warning.
Condition Name: (Content)
|
Overview
Safinamide is a Acetylcholine release inhibitor, Adrenergic receptor agonist that is FDA approved for the (type of indication of drug) of a list of indications, separated by commas.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include a list of adverse reactions, separated by commas..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
CONTRAINDICATIONS
Warnings
|
Warning Title
See full prescribing information for complete Boxed Warning.
Condition Name: (Content)
|
Conidition 1
(Description)
Conidition 2
(Description)
Conidition 3
(Description)
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Condition 2
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
(Description)
Drug Interactions
- Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Pregnancy Category C
- There are no adequate and well-controlled studies of XADAGO in pregnant women. In animals, developmental toxicity, including teratogenic effects, was observed when safinamide was administered during pregnancy at clinically relevant doses. Developmental toxicity was observed at safinamide doses lower than those used clinically when safinamide was administered during pregnancy in combination with levodopa/carbidopa. XADAGO should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- In an embryofetal development study in rats, oral administration of safinamide (0, 50, 100, or 150 mg/kg/day) throughout organogenesis resulted in dose-related increases in fetal abnormalities (primarily urogenital malformations) at all doses. The lowest dose tested is approximately 5 times the maximum recommended human dose (MRHD) of 100 mg on a body surface area (mg/m 2) basis. In a combination embryofetal development study of safinamide and levodopa (LD)/carbidopa (CD) in rats (80/20 mg/kg/day LD/CD in combination with 0, 25, 50, or 100 mg/kg/day safinamide or 100 mg/kg/day safinamide alone) increased incidences of fetal visceral and skeletal malformations and variations were observed at all doses of safinamide in combination with CD/LD and with safinamide alone. The lowest dose of safinamide tested (25 mg/kg/day) is approximately 2 times the MRHD on a mg/m 2 basis.
- In embryofetal development studies in rabbits, no developmental toxicity was observed at up to the highest oral dose of safinamide tested (100 mg/kg/day). However, when safinamide (4, 12, or 40 mg/kg/day) was administered throughout organogenesis in a combination study of safinamide with LD/CD (80/20 mg/kg/day LD/CD) there was an increased incidence of embryofetal death and cardiac and skeletal malformations, compared to LD/CD alone. A no-effect dose for safinamide was not established; the lowest effect dose of safinamide tested (4 mg/kg/day) is less than the MRHD on a mg/m 2 basis.
- In a rat pre- and postnatal development study, oral administration of safinamide (0, 4, 12.5, or 37.5 mg/kg/day) throughout pregnancy and lactation resulted in skin discoloration of the offspring, presumed to be due to hepatobiliary toxicity, at the mid and high doses and decreased body weight and increased postnatal mortality in offspring at the highest dose tested. The no effect dose (4 mg/kg/day) is less than the MRHD on a mg/m 2 basis.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Safinamide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Safinamide during labor and delivery.
Nursing Mothers
- Skin discoloration, presumed to be caused by hyperbilirubinemia resulting from hepatobiliary toxicity, was observed in rat pups indirectly exposed to safinamide through the milk during the lactation period. It is not known whether this drug is present in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from safinamide, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- The safety and effectiveness of XADAGO in pediatric patients have not been established. No juvenile toxicity studies have been performed in animals.
Geriatic Use
- Of the 1516 subjects exposed to XADAGO in clinical studies, 38% were 65 and over, while 4% were 75 and over. No overall differences in safety or effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Gender
There is no FDA guidance on the use of Safinamide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Safinamide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Safinamide in patients with renal impairment.
Hepatic Impairment
- XADAGO plasma concentrations are increased in patients with hepatic impairment.
- In patients with moderate hepatic impairment (Child-Pugh B: 7-9), the maximum recommended dosage of XADAGO is 50 mg once daily. XADAGO has not been studied in patients with severe hepatic impairment (Child-Pugh C: 10-15), and is contraindicated in these patients. If patients progress from moderate to severe hepatic impairment, treatment with XADAGO should be stopped.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Safinamide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Safinamide in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
- Give at the same time each day, with or without meals.
- Avoid consumption of foods high in tyramine.
Monitoring
- An increase in the total daily "on" time without troublesome dyskinesia associated with Parkinson disease may indicate efficacy.
- Hypertension: New onset of or lack of blood pressure control in hypertensive patients, especially in patients receiving concomitant sympathomimetics, including decongestants and cold remedies.
- New or increased impulse control or compulsive behaviors: Such as gambling, sexual, or spending urges, which may be unrecognizable by the patient as abnormal.
- Visual changes: Periodically in patients with history of retinal or macular degeneration, uveitis, inherited retinal conditions, family history of hereditary retinal disease, albinism, rentinis pigmentosa or any active retinopathy (eg diabetic retinopathy).
IV Compatibility
There is limited information regarding the compatibility of Safinamide and IV administrations.
Overdosage
- There is no human experience with XADAGO overdose.
- There is no known antidote to XADAGO nor any specific treatment for XADAGO overdose. If an important overdose occurs, XADAGO treatment should be discontinued and supportive treatment should be administered as clinically indicated. In cases of overdose with XADAGO, dietary tyramine restriction should be observed for several weeks.
- The Poison Control Center should be called at 1-800-222-1222 for the most current treatment guidelines.
Pharmacology
Mechanism of Action
- The precise mechanism by which XADAGO exerts its effect in Parkinson's disease is unknown. XADAGO is an inhibitor of monoamine oxidase B (MAO-B). Inhibition of MAO-B activity, by blocking the catabolism of dopamine, is thought to result in an increase in dopamine levels and a subsequent increase in dopaminergic activity in the brain.
Structure

Pharmacodynamics
- XADAGO inhibits monoamine oxidase B (MAO-B), with more than 1000-fold selectivity over MAO-A. In clinical studies, complete inhibition (>90%) of MAO-B was measured at doses > 20 mg.
Tyramine Challenge Test
- In an oral tyramine challenge study, XADAGO produced a distinct but relatively small increase in tyramine sensitivity to increase blood pressure. The results suggest that XADAGO at a dose of 50 mg or 100 mg is relatively selective for inhibiting MAO-B and can be used without dietary tyramine restriction. Relative selectivity of XADAGO for inhibiting MAO-B decreases above the highest recommended daily dosage (100 mg).
Cardiac Electrophysiology
- The effect of XADAGO on the QTc interval was evaluated in a randomized placebo and positive controlled double-blind, multiple-dose parallel thorough QTc study in 240 healthy subjects. At a dose of 350 mg (3.5 times the maximum recommended dosage), XADAGO did not prolong the QTc interval.
Pharmacokinetics
- Pharmacokinetics of safinamide is linear over a range of 50 mg to 300 mg (3 times the maximum recommended daily dose). Steady state is reached within 5 to 6 days.
Absorption
- After single and multiple oral dosing under fasting conditions, Tmax of safinamide ranges from 2 to 3 hours. Absolute bioavailability of safinamide is 95% after oral administration, and first pass metabolism is negligible. A slight delay in Tmax was observed in the fed state relative to the fasted condition, but there was no effect on safinamide AUC 0−∞ and Cmax.
Distribution
- The volume of distribution (Vss) is approximately 165 L, indicating extensive extravascular distribution. Safinamide is not highly protein bound (unbound fraction is 11 to 12%).
Metabolism and Excretion
- In humans, safinamide is almost exclusively eliminated via metabolism (~5% of the drug is eliminated unchanged, mainly in urine), through three main metabolic pathways. One pathway involves hydrolytic oxidation of the amide moiety leading to the primary metabolite 'safinamide acid' (NW-1153). Another pathway is oxidative cleavage of the ether bond forming 'O- debenzylated safinamide' (NW-1199). Finally, the 'N-dealkylated acid' (NW-1689) is formed by oxidative cleavage of the amine bond of either safinamide or the primary safinamide acid metabolite (NW-1153). The 'N-dealkylated acid' (NW-1689) undergoes further conjugation with glucuronic acid yielding its acyl glucuronide. NW-1689 is the main circulating metabolite in human plasma, exceeding the exposure of the parent (161% of parent). NW-1689 AG and NW-1153 account for about 18% and 11% of the parent drug exposure, respectively. None of the metabolites has pharmacological activity.
- Safinamide is predominantly metabolized by non-microsomal enzymes (cytosolic amidases/MAO-A); CYP3A4 and other CYP iso-enzymes play only a minor role in its overall biotransformation.
- The total clearance of safinamide was determined to be 4.6 L/h. Terminal half-life is 20-26 h. The primary route of excretion is through the kidney (76% of safinamide dose recovered in the urine, primarily in the form of inactive metabolites).
Specific Populations
- Age: Geriatric Population: There are limited clinical data on the use of XADAGO in the elderly (>75 years). These data suggest that the pharmacokinetics of safinamide is not affected by age.
- Race: The pharmacokinetics of safinamide is not influenced by race.
- Sex: The pharmacokinetics of safinamide is not influenced by sex.
- Hepatic Impairment: The disposition of XADAGO was assessed in subjects with mild and moderate hepatic impairment and compared with subjects with normal hepatic function. A marginal increase in the exposure of safinamide (approximately 30% increase in AUC) was observed in subjects with mild hepatic impairment (Child-Pugh A). In subjects with moderate hepatic impairment (Child-Pugh B), exposure to safinamide was increased by about 80% (CI: 154-215%). XADAGO has not been studied in patients with severe hepatic impairment (Child-Pugh C).
- Renal Impairment: The effect of renal impairment on safinamide pharmacokinetics was investigated in an open-label, parallel-group, single oral dose study in subjects with moderate renal impairment, severe renal impairment, or normal renal function. The pharmacokinetics of safinamide was not affected by impaired renal function.
Drug Interaction Studies
- In Vitro Studies: In vitro metabolism studies indicate no meaningful inhibition or induction of Cytochrome P450 (CYP) based enzymes by safinamide and its major metabolites at concentrations that are relevant for dosing. Safinamide or its major metabolites at clinically relevant concentrations are not inhibitors of MAO-A, levodopa decarboxylase or aldehyde dehydrogenase enzymes.
- Safinamide is not a substrate of P-gp. Safinamide and its metabolites did not inhibit P-gp or other transporters OCT2, OATP1B1, OATP1B3, BSEP, OAT1/3/4. Safinamide and NW-1689 may inhibit BCRP at the 100 mg dose.
- In Vivo Studies: Dedicated drug-drug interactions studies conducted with ketoconazole, levodopa (LD) and CYP1A2 and CYP3A4 substrates (caffeine and midazolam, respectively) did not demonstrate any clinically significant effects on the pharmacokinetic profile of XADAGO, or on the pharmacokinetic profile of co-administered levodopa or CYP1A2 and CYP3A4 substrates.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
- In carcinogenicity studies in mice and rats, safinamide was administered at oral doses of 0, 50, 100 and 200 mg/kg/day, and 0, 25, 50 and 100 mg/kg/day, respectively, for 2 years. The highest doses tested in both species were approximately 10 times the maximum recommended human dose (MRHD) of 100 mg/day on a body surface area (mg/m 2) basis. No evidence of tumorigenic potential was observed in either species.
Mutagenesis
- Safinamide was negative for genotoxicity in in vitro (Ames, mouse lymphoma) and in vivo (mouse micronucleus) assays.
Impairment of Fertility
- In a rat fertility study in which males and females were orally administered safinamide (0, 50, 100, 150 mg/kg/day) prior to and during mating and continuing through early pregnancy in females, adverse effects on reproductive function were observed in both males (sperm abnormalities) and females (decreased corpora lutea, increased pre-implantation loss). The no-effect dose for reproductive toxicity (50 mg/kg/day) is approximately 5 times the MRHD on a mg/m 2 basis.
Animal Toxicology and /or Pharmacology
Retinal Pathology in Rats
- Degeneration and loss of photoreceptor cells were observed in the retina of both albino and pigmented rats at plasma exposures lower than that in humans at the maximum recommended human dose of 100 mg/kg/day. The findings were dose- and time-dependent and progressed from minimal loss to severe outer nuclear cell layer loss after one year of oral dosing with safinamide. In a two year study, total retinal atrophy and scarring and lens opacities (cataracts) were seen at all oral doses tested (0, 25, 50, and 100 mg/kg/day).
- In a study in rats dosed orally with safinamide alone or in combination with pramipexole, pramipexole, at a dose (25 mg/kg/day) that did not cause retinal changes, exacerbated the retinal pathology caused by safinamide alone (50 mg/kg/day) in both pigmented and albino rats.
- Investigative studies were not able to identify a mechanism underlying the retinal toxicity; the relevance to humans is unknown.
Clinical Studies
Adjunctive Treatment in Patients with Parkinson's Disease Experiencing OFF Time on a Stable Dose of Levodopa.
- Two double-blind, placebo-controlled, multi-national, 24-week studies (Study 1 and Study 2) were conducted in PD patients experiencing "OFF" Time during treatment with carbidopa/levodopa and other PD medications, e.g., dopamine agonists, catechol-O-methyl transferase (COMT) inhibitors, anticholinergics, and/or amantadine. In both studies, the primary measure of effectiveness was the change from baseline in total daily "ON" Time without troublesome dyskinesia (i.e., "ON" Time without dyskinesia plus "ON" Time with non- troublesome dyskinesia), based on 18-hour diaries completed by patients for at least 3 days before each of the scheduled visits. Secondary endpoints included "OFF" Time during the diary period and reduction in Uniform Parkinson's Disease Rating Scale (UPDRS) Part III (motor examination).
- In Study 1, patients (n=645) were randomized equally to treatment with XADAGO 50 mg/day (n=217 patients), XADAGO 100 mg/day (n=216 patients), or placebo (n=212 patients), and had at least one post-baseline assessment of "ON" Time.
- The percentages of patients taking stable doses of other classes of PD medications, in addition to levodopa/decarboxylase inhibitor, were: dopamine agonists (61%), COMT inhibitors (24%), anticholinergics (37%), and amantadine (14%). Use of MAO inhibitors was prohibited. The average daily dosage of levodopa was 630 mg. The mean duration of Parkinson's disease was approximately 8 years.
- In Study 1, XADAGO 50 mg/day and 100 mg/day significantly increased "ON" Time compared to placebo (Table 2). The increase in "ON" Time without troublesome dyskinesia was accompanied by a similar significant reduction in "OFF" Time and a reduction in Unified Parkinson's Disease Rating Scale Part III (UPDRS III) scores assessed during "ON" Time (Table 3). Improvement in "ON Time" occurred without an increase in troublesome dyskinesia.


- The effect of XADAGO 100 mg on "ON" Time was only slightly numerically greater than the effect of XADAGO 50 mg. In addition, the time course of improvement in total daily "ON" Time was similar between both doses (Figure 1). The time course of improvement in total daily "ON" Time showed numerically greater improvement with both XADAGO 50mg and 100 mg compared to placebo, at all post-baseline timepoints (Figure 1).

- Figure 2 shows the empirical cumulative distribution functions (CDF) for the change from baseline to Week 24 in total daily "ON" Time in Study 1. The cumulative percentage of patients with a change in "ON" Time was similar for the XADAGO 50 mg and 100 mg groups. The cumulative percentage of patients with an increase in "ON" Time is higher for both XADAGO 50 mg and 100 mg treated patients than for placebo patients.

- Patients who dropped out of the study because of an adverse reaction, lack of efficacy, non-compliance, or withdrawal of consent were treated as treatment failures and assumed to have the smallest change from baseline among all patients. The failure rates are 6.1%, 5.6%, and 6.9% for the placebo group, XADAGO 50 mg/day group, and XADAGO 100 mg/day group, respectively.
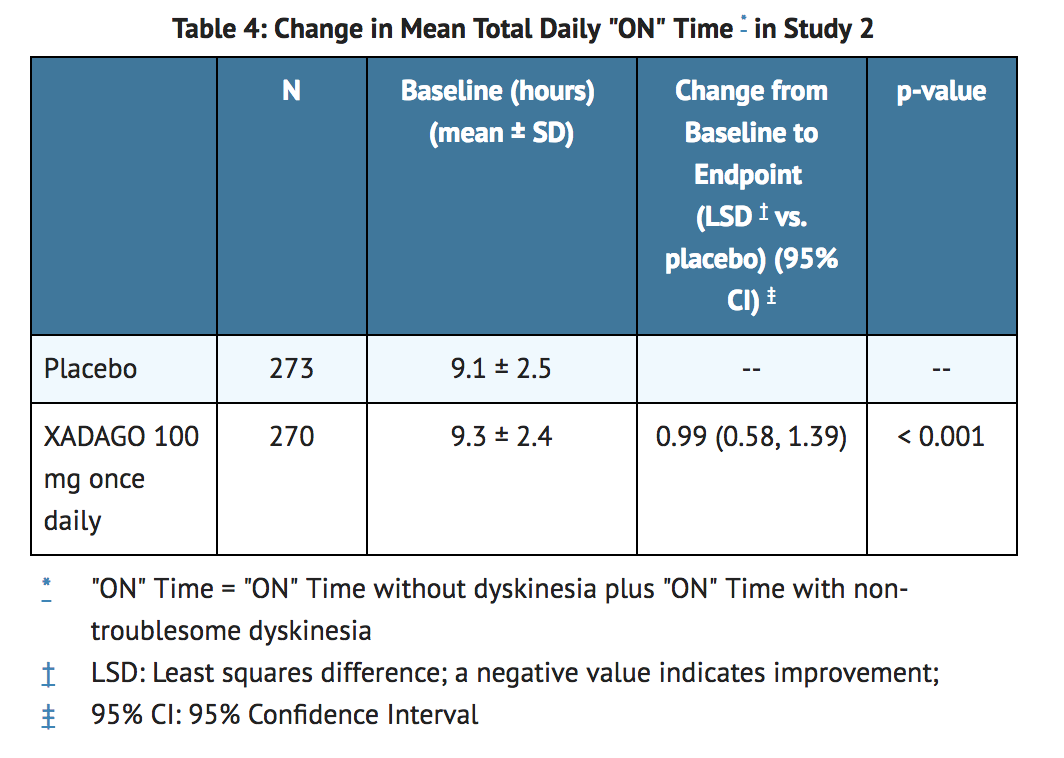
- In Study 2, patients (n=549) were randomized to treatment with XADAGO 100 mg daily (n=274 patients) or placebo (n=275 patients) for up to 24 weeks. The percentages of patients taking stable doses of other classes of PD medication, in addition to levodopa/decarboxylase inhibitor, were: dopamine agonists (74%), COMT inhibitors (18%), anticholinergics (17%), and amantadine (30%). Use of MAO inhibitors was prohibited. The average daily dosage of levodopa was 777 mg. The mean duration of Parkinson's disease was approximately 9 years.
- In Study 2, XADAGO was significantly better than placebo for increasing "ON" Time (Table 4). The observed increase in "ON" Time without troublesome dyskinesia was accompanied by a reduction in "OFF" Time of similar magnitude and a reduction in UPDRS III score (assessed during "ON" Time). The time course of effect was similar to that showed in the above figure for Study 1. As in Study 1, the increase in "ON" Time without troublesome dyskinesia was accompanied by a similar significant reduction in "OFF" Time and a reduction in Unified Parkinson's Disease Rating Scale Part III (UPDRS III) scores assessed during "ON" Time (Table 5).

- The time course of improvement in total daily "ON" Time showed numerically greater improvement with XADAGO 100 mg compared to placebo at all post-baseline timepoints (Figure 3).

- Figure 4 shows the empirical cumulative distribution functions (CDF) for the change from baseline to Week 24 in total daily "ON" Time in Study 2. The cumulative percentage of patients with an increase in "ON" Time treated with XADAGO 50 mg to 100 mg is higher than for placebo patients.

How Supplied
- 50 mg (orange to copper colored with metallic gloss, round film-coated, biconcave shaped tablet embossed with "50" on one side; approximately 7 mm in diameter).

- 100 mg (orange to copper colored with metallic gloss, round film-coated, biconcave shaped tablet embossed with "100" on one side; approximately 9 mm in diameter).

Storage
- Store at 25°C (77°F); excursions permitted between 15°C to 30°C (59°F to 86°F).
Images
Drug Images
{{#ask: Page Name::Safinamide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel






{{#ask: Label Page::Safinamide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Hypertension
- Advise patients that treatment with recommended doses of XADAGO may be associated with elevations of blood pressure or onset of hypertension. Tell patients who experience elevation of blood pressure while taking XADAGO to contact their healthcare provider.
- Explain the risk of using higher than recommended daily doses of XADAGO, and provide a brief description of the tyramine associated hypertensive reaction.
- Advise patients to avoid certain foods (e.g., aged cheese) containing a very large amount of tyramine while taking recommended doses of XADAGO because of the potential for large increases in blood pressure. If patients eat foods very rich in tyramine and do not feel well soon after eating, they should contact their healthcare provider.
Serotonin Syndrome
- Tell patients to inform their physician if they are taking, or planning to take, any prescription or over-thecounter drugs, especially antidepressants and over-the-counter cold medications, because there is a potential for interaction with XADAGO. Because patients should not use meperidine or certain other analgesics with XADAGO, they should contact their healthcare provider before taking new medications including antidepressants, analgesics, and prescription or nonprescription decongestants.
Falling Asleep During Activities of Daily Living and Somnolence
- Inform patients about the potential for sedating effects associated with XADAGO and other dopaminergic medications, including somnolence and particularly to the possibility of falling asleep while engaged in activities of daily living. Because somnolence can be a frequent adverse reaction with potentially serious consequences, patients should not operate a motor vehicle or engage in other potentially dangerous activities until they have gained sufficient experience with XADAGO.
- Advise patients that if increased somnolence or new episodes of falling asleep during activities of daily living (e.g., watching television, passenger in a car, etc.) are experienced at any time during treatment, they should not drive or participate in potentially dangerous activities until they have contacted their physician. Patients should not drive, operate machinery, or work at heights during treatment if they have previously experienced somnolence and/or have fallen asleep without warning prior to use of XADAGO.
- Because of possible additive effects, advise patients about the potential for increased somnolence when patients are taking other sedating medications, alcohol, or other central nervous system depressants (e.g., benzodiazepines, antipsychotics, antidepressants) in combination with XADAGO.
Dyskinesia
- Advise patients taking XADAGO as adjunct to levodopa that there is a possibility of dyskinesia or increased dyskinesia.
Hallucinations / Psychotic Behavior
Inform patients that hallucinations or other manifestations of psychotic behavior can occur when taking XADAGO. Advise patients that, if they have a major psychotic disorder, that XADAGO should not ordinarily be used because of the risk of exacerbating the psychosis. Patients with a major psychotic disorder should also be aware that many treatments for psychosis may decrease the effectiveness of XADAGO.
Impulse Control/Compulsive Behaviors
- Advise patients that they may experience intense urges to gamble, increased sexual urges, other intense urges, and the inability to control these urges while taking XADAGO. Although it is not proven that the medications caused these events, these urges were reported to have stopped in some cases when the dose was reduced or the medication was stopped. Prescribers should ask patients about the development of new or increased gambling urges, sexual urges, or other urges while being treated with XADAGO. Patients should inform their physician if they experience these urges while taking XADAGO.
Withdrawal-Emergent Hyperpyrexia and Confusion
- Tell patients to contact their healthcare provider if they wish to discontinue XADAGO and seek guidance for tapering XADAGO instead of abruptly discontinuing XADAGO.
Missing Dose
- Instruct patients to take XADAGO as prescribed. If a dose is missed, instruct patients to take the next dose at the usual time on the following day.
Concomitant Medications
- Advise patients to inform their physicians if they are taking, or plan to take, any prescription or over-the-counter medications because of a potential for interactions.

Precautions with Alcohol
Alcohol-Safinamide interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Xadago
Look-Alike Drug Names
There is limited information regarding Safinamide Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "Summary of the risk management plan (RMP) for Xadago (safinamide)" (PDF). European Medicines Agency. January 2015.