Safinamide: Difference between revisions
No edit summary |
No edit summary |
||
| Line 302: | Line 302: | ||
|PK=(Description) | |PK=(Description) | ||
|nonClinToxic=(Description) | |nonClinToxic=(Description) | ||
|clinicalStudies====== | |clinicalStudies======Adjunctive Treatment in Patients with Parkinson's Disease Experiencing OFF Time on a Stable Dose of Levodopa.===== | ||
( | *Two double-blind, placebo-controlled, multi-national, 24-week studies (Study 1 and Study 2) were conducted in PD patients experiencing "OFF" Time during treatment with carbidopa/levodopa and other PD medications, e.g., dopamine agonists, catechol-O-methyl transferase (COMT) inhibitors, anticholinergics, and/or amantadine. In both studies, the primary measure of effectiveness was the change from baseline in total daily "ON" Time without troublesome dyskinesia (i.e., "ON" Time without dyskinesia plus "ON" Time with non- troublesome dyskinesia), based on 18-hour diaries completed by patients for at least 3 days before each of the scheduled visits. Secondary endpoints included "OFF" Time during the diary period and reduction in Uniform Parkinson's Disease Rating Scale (UPDRS) Part III (motor examination). | ||
==== | *In Study 1, patients (n=645) were randomized equally to treatment with XADAGO 50 mg/day (n=217 patients), XADAGO 100 mg/day (n=216 patients), or placebo (n=212 patients), and had at least one post-baseline assessment of "ON" Time. | ||
( | *The percentages of patients taking stable doses of other classes of PD medications, in addition to levodopa/decarboxylase inhibitor, were: dopamine agonists (61%), COMT inhibitors (24%), anticholinergics (37%), and amantadine (14%). Use of MAO inhibitors was prohibited. The average daily dosage of levodopa was 630 mg. The mean duration of Parkinson's disease was approximately 8 years. | ||
=== | *In Study 1, XADAGO 50 mg/day and 100 mg/day significantly increased "ON" Time compared to placebo (Table 2). The increase in "ON" Time without troublesome dyskinesia was accompanied by a similar significant reduction in "OFF" Time and a reduction in Unified Parkinson's Disease Rating Scale Part III (UPDRS III) scores assessed during "ON" Time (Table 3). Improvement in "ON Time" occurred without an increase in troublesome dyskinesia. | ||
[[image:Safinamide_Clinical_Studies_Table_1.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | |||
[[image:Safinamide_Clinical_Studies_Table_2.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | |||
*The effect of XADAGO 100 mg on "ON" Time was only slightly numerically greater than the effect of XADAGO 50 mg. In addition, the time course of improvement in total daily "ON" Time was similar between both doses (Figure 1). The time course of improvement in total daily "ON" Time showed numerically greater improvement with both XADAGO 50mg and 100 mg compared to placebo, at all post-baseline timepoints (Figure 1). | |||
[[image:Safinamide_Clinical_Studies_Figure_1.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | |||
*Figure 2 shows the empirical cumulative distribution functions (CDF) for the change from baseline to Week 24 in total daily "ON" Time in Study 1. The cumulative percentage of patients with a change in "ON" Time was similar for the XADAGO 50 mg and 100 mg groups. The cumulative percentage of patients with an increase in "ON" Time is higher for both XADAGO 50 mg and 100 mg treated patients than for placebo patients. | |||
[[image:Safinamide_Clinical_Studies_Figure_2.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | |||
*Patients who dropped out of the study because of an adverse reaction, lack of efficacy, non-compliance, or withdrawal of consent were treated as treatment failures and assumed to have the smallest change from baseline among all patients. The failure rates are 6.1%, 5.6%, and 6.9% for the placebo group, XADAGO 50 mg/day group, and XADAGO 100 mg/day group, respectively. | |||
*In Study 2, patients (n=549) were randomized to treatment with XADAGO 100 mg daily (n=274 patients) or placebo (n=275 patients) for up to 24 weeks. The percentages of patients taking stable doses of other classes of PD medication, in addition to levodopa/decarboxylase inhibitor, were: dopamine agonists (74%), COMT inhibitors (18%), anticholinergics (17%), and amantadine (30%). Use of MAO inhibitors was prohibited. The average daily dosage of levodopa was 777 mg. The mean duration of Parkinson's disease was approximately 9 years. | |||
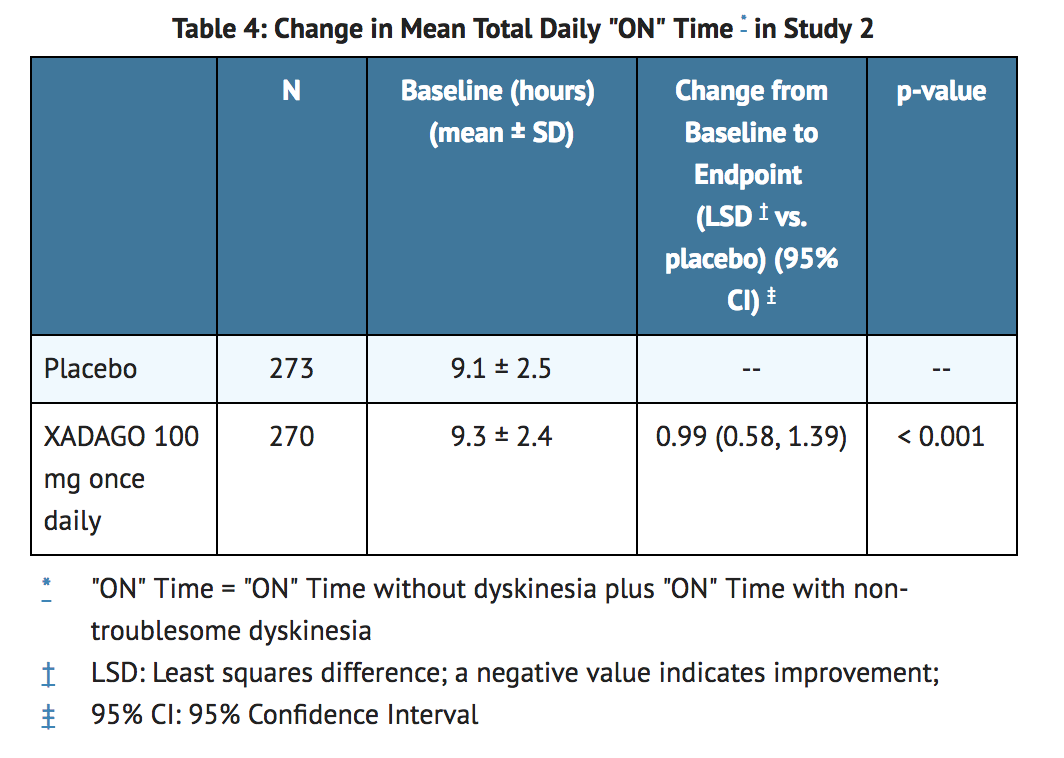
*In Study 2, XADAGO was significantly better than placebo for increasing "ON" Time (Table 4). The observed increase in "ON" Time without troublesome dyskinesia was accompanied by a reduction in "OFF" Time of similar magnitude and a reduction in UPDRS III score (assessed during "ON" Time). The time course of effect was similar to that showed in the above figure for Study 1. As in Study 1, the increase in "ON" Time without troublesome dyskinesia was accompanied by a similar significant reduction in "OFF" Time and a reduction in Unified Parkinson's Disease Rating Scale Part III (UPDRS III) scores assessed during "ON" Time (Table 5). | |||
[[image:Safinamide_Clinical_Studies_Table_3.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | |||
[[image:Safinamide_Clinical_Studies_Table_4.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | |||
*The time course of improvement in total daily "ON" Time showed numerically greater improvement with XADAGO 100 mg compared to placebo at all post-baseline timepoints (Figure 3). | |||
[[image:Safinamide_Clinical_Studies_Figure_3.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | |||
*Figure 4 shows the empirical cumulative distribution functions (CDF) for the change from baseline to Week 24 in total daily "ON" Time in Study 2. The cumulative percentage of patients with an increase in "ON" Time treated with XADAGO 50 mg to 100 mg is higher than for placebo patients. | |||
[[image:Safinamide_Clinical_Studies_Figure_4.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | |||
|howSupplied=*50 mg (orange to copper colored with metallic gloss, round film-coated, biconcave shaped tablet embossed with "50" on one side; approximately 7 mm in diameter). | |howSupplied=*50 mg (orange to copper colored with metallic gloss, round film-coated, biconcave shaped tablet embossed with "50" on one side; approximately 7 mm in diameter). | ||
Revision as of 15:41, 30 July 2018
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Yashasvi Aryaputra[2];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Warning Title
See full prescribing information for complete Boxed Warning.
Condition Name: (Content)
|
Overview
Safinamide is a Acetylcholine release inhibitor, Adrenergic receptor agonist that is FDA approved for the (type of indication of drug) of a list of indications, separated by commas.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include a list of adverse reactions, separated by commas..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
CONTRAINDICATIONS
Warnings
|
Warning Title
See full prescribing information for complete Boxed Warning.
Condition Name: (Content)
|
Conidition 1
(Description)
Conidition 2
(Description)
Conidition 3
(Description)
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Condition 2
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
(Description)
Drug Interactions
- Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
(Description)
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Safinamide in women who are pregnant.
Labor and Delivery
(Description)
Nursing Mothers
(Description)g
Pediatric Use
(Description)
Geriatic Use
(Description)
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
There is limited information regarding the compatibility of Safinamide and IV administrations.
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
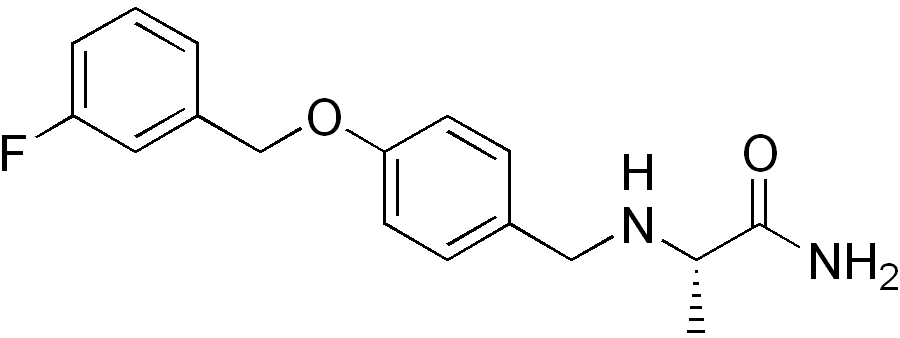
| |
Safinamide
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
(Description)
Structure
(Description with picture)
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
Adjunctive Treatment in Patients with Parkinson's Disease Experiencing OFF Time on a Stable Dose of Levodopa.
- Two double-blind, placebo-controlled, multi-national, 24-week studies (Study 1 and Study 2) were conducted in PD patients experiencing "OFF" Time during treatment with carbidopa/levodopa and other PD medications, e.g., dopamine agonists, catechol-O-methyl transferase (COMT) inhibitors, anticholinergics, and/or amantadine. In both studies, the primary measure of effectiveness was the change from baseline in total daily "ON" Time without troublesome dyskinesia (i.e., "ON" Time without dyskinesia plus "ON" Time with non- troublesome dyskinesia), based on 18-hour diaries completed by patients for at least 3 days before each of the scheduled visits. Secondary endpoints included "OFF" Time during the diary period and reduction in Uniform Parkinson's Disease Rating Scale (UPDRS) Part III (motor examination).
- In Study 1, patients (n=645) were randomized equally to treatment with XADAGO 50 mg/day (n=217 patients), XADAGO 100 mg/day (n=216 patients), or placebo (n=212 patients), and had at least one post-baseline assessment of "ON" Time.
- The percentages of patients taking stable doses of other classes of PD medications, in addition to levodopa/decarboxylase inhibitor, were: dopamine agonists (61%), COMT inhibitors (24%), anticholinergics (37%), and amantadine (14%). Use of MAO inhibitors was prohibited. The average daily dosage of levodopa was 630 mg. The mean duration of Parkinson's disease was approximately 8 years.
- In Study 1, XADAGO 50 mg/day and 100 mg/day significantly increased "ON" Time compared to placebo (Table 2). The increase in "ON" Time without troublesome dyskinesia was accompanied by a similar significant reduction in "OFF" Time and a reduction in Unified Parkinson's Disease Rating Scale Part III (UPDRS III) scores assessed during "ON" Time (Table 3). Improvement in "ON Time" occurred without an increase in troublesome dyskinesia.


- The effect of XADAGO 100 mg on "ON" Time was only slightly numerically greater than the effect of XADAGO 50 mg. In addition, the time course of improvement in total daily "ON" Time was similar between both doses (Figure 1). The time course of improvement in total daily "ON" Time showed numerically greater improvement with both XADAGO 50mg and 100 mg compared to placebo, at all post-baseline timepoints (Figure 1).

- Figure 2 shows the empirical cumulative distribution functions (CDF) for the change from baseline to Week 24 in total daily "ON" Time in Study 1. The cumulative percentage of patients with a change in "ON" Time was similar for the XADAGO 50 mg and 100 mg groups. The cumulative percentage of patients with an increase in "ON" Time is higher for both XADAGO 50 mg and 100 mg treated patients than for placebo patients.

- Patients who dropped out of the study because of an adverse reaction, lack of efficacy, non-compliance, or withdrawal of consent were treated as treatment failures and assumed to have the smallest change from baseline among all patients. The failure rates are 6.1%, 5.6%, and 6.9% for the placebo group, XADAGO 50 mg/day group, and XADAGO 100 mg/day group, respectively.
- In Study 2, patients (n=549) were randomized to treatment with XADAGO 100 mg daily (n=274 patients) or placebo (n=275 patients) for up to 24 weeks. The percentages of patients taking stable doses of other classes of PD medication, in addition to levodopa/decarboxylase inhibitor, were: dopamine agonists (74%), COMT inhibitors (18%), anticholinergics (17%), and amantadine (30%). Use of MAO inhibitors was prohibited. The average daily dosage of levodopa was 777 mg. The mean duration of Parkinson's disease was approximately 9 years.
- In Study 2, XADAGO was significantly better than placebo for increasing "ON" Time (Table 4). The observed increase in "ON" Time without troublesome dyskinesia was accompanied by a reduction in "OFF" Time of similar magnitude and a reduction in UPDRS III score (assessed during "ON" Time). The time course of effect was similar to that showed in the above figure for Study 1. As in Study 1, the increase in "ON" Time without troublesome dyskinesia was accompanied by a similar significant reduction in "OFF" Time and a reduction in Unified Parkinson's Disease Rating Scale Part III (UPDRS III) scores assessed during "ON" Time (Table 5).

- The time course of improvement in total daily "ON" Time showed numerically greater improvement with XADAGO 100 mg compared to placebo at all post-baseline timepoints (Figure 3).

- Figure 4 shows the empirical cumulative distribution functions (CDF) for the change from baseline to Week 24 in total daily "ON" Time in Study 2. The cumulative percentage of patients with an increase in "ON" Time treated with XADAGO 50 mg to 100 mg is higher than for placebo patients.

How Supplied
- 50 mg (orange to copper colored with metallic gloss, round film-coated, biconcave shaped tablet embossed with "50" on one side; approximately 7 mm in diameter).

- 100 mg (orange to copper colored with metallic gloss, round film-coated, biconcave shaped tablet embossed with "100" on one side; approximately 9 mm in diameter).

Storage
- Store at 25°C (77°F); excursions permitted between 15°C to 30°C (59°F to 86°F).
Images
Drug Images
{{#ask: Page Name::Safinamide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel






{{#ask: Label Page::Safinamide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Hypertension
- Advise patients that treatment with recommended doses of XADAGO may be associated with elevations of blood pressure or onset of hypertension. Tell patients who experience elevation of blood pressure while taking XADAGO to contact their healthcare provider.
- Explain the risk of using higher than recommended daily doses of XADAGO, and provide a brief description of the tyramine associated hypertensive reaction.
- Advise patients to avoid certain foods (e.g., aged cheese) containing a very large amount of tyramine while taking recommended doses of XADAGO because of the potential for large increases in blood pressure. If patients eat foods very rich in tyramine and do not feel well soon after eating, they should contact their healthcare provider.
Serotonin Syndrome
- Tell patients to inform their physician if they are taking, or planning to take, any prescription or over-thecounter drugs, especially antidepressants and over-the-counter cold medications, because there is a potential for interaction with XADAGO. Because patients should not use meperidine or certain other analgesics with XADAGO, they should contact their healthcare provider before taking new medications including antidepressants, analgesics, and prescription or nonprescription decongestants.
Falling Asleep During Activities of Daily Living and Somnolence
- Inform patients about the potential for sedating effects associated with XADAGO and other dopaminergic medications, including somnolence and particularly to the possibility of falling asleep while engaged in activities of daily living. Because somnolence can be a frequent adverse reaction with potentially serious consequences, patients should not operate a motor vehicle or engage in other potentially dangerous activities until they have gained sufficient experience with XADAGO.
- Advise patients that if increased somnolence or new episodes of falling asleep during activities of daily living (e.g., watching television, passenger in a car, etc.) are experienced at any time during treatment, they should not drive or participate in potentially dangerous activities until they have contacted their physician. Patients should not drive, operate machinery, or work at heights during treatment if they have previously experienced somnolence and/or have fallen asleep without warning prior to use of XADAGO.
- Because of possible additive effects, advise patients about the potential for increased somnolence when patients are taking other sedating medications, alcohol, or other central nervous system depressants (e.g., benzodiazepines, antipsychotics, antidepressants) in combination with XADAGO.
Dyskinesia
- Advise patients taking XADAGO as adjunct to levodopa that there is a possibility of dyskinesia or increased dyskinesia.
Hallucinations / Psychotic Behavior
Inform patients that hallucinations or other manifestations of psychotic behavior can occur when taking XADAGO. Advise patients that, if they have a major psychotic disorder, that XADAGO should not ordinarily be used because of the risk of exacerbating the psychosis. Patients with a major psychotic disorder should also be aware that many treatments for psychosis may decrease the effectiveness of XADAGO.
Impulse Control/Compulsive Behaviors
- Advise patients that they may experience intense urges to gamble, increased sexual urges, other intense urges, and the inability to control these urges while taking XADAGO. Although it is not proven that the medications caused these events, these urges were reported to have stopped in some cases when the dose was reduced or the medication was stopped. Prescribers should ask patients about the development of new or increased gambling urges, sexual urges, or other urges while being treated with XADAGO. Patients should inform their physician if they experience these urges while taking XADAGO.
Withdrawal-Emergent Hyperpyrexia and Confusion
- Tell patients to contact their healthcare provider if they wish to discontinue XADAGO and seek guidance for tapering XADAGO instead of abruptly discontinuing XADAGO.
Missing Dose
- Instruct patients to take XADAGO as prescribed. If a dose is missed, instruct patients to take the next dose at the usual time on the following day.
Concomitant Medications
- Advise patients to inform their physicians if they are taking, or plan to take, any prescription or over-the-counter medications because of a potential for interactions.

Precautions with Alcohol
Alcohol-Safinamide interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Xadago
Look-Alike Drug Names
There is limited information regarding Safinamide Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.