Rhabdomyoma
|
Rhabdomyoma Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Rhabdomyoma On the Web |
|
American Roentgen Ray Society Images of Rhabdomyoma |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Nima Nasiri, M.D.[2]
Synonyms and keywords: Rhabdomyomatous neoplasm; Adult rhabdomyoma; Genital rhabdomyoma; Fetal rhabdomyoma; Rhabdomyomatous mesenchymal hamartomas
Overview
Rhabdomyoma is a benign tumor of striated muscle. Rhabdomyomas are rare and can be classified into cardiac type and extracardiac type. The most common primary benign pediatric tumor of the heart is cardiac rhabdomyoma which can be seen mainly in fetal life and children, second most common primary benign cardiac tumor in children is fibroma. Most tumors regress spontaneously, prognosis depends on the location of tumor and size. Cardiac rhabdomyoma is strongly associated with tuberous sclerosis. Cardiac rhabdomyoma is seen almost always in the pediatric age group and is associated with tuberous sclerosis, neurofibromatosis, and sebaceous adenomas. Extracardiac rhabdomyoma can be divided into three groups (adult, fetal, and genital types) with distinct clinical and morphological differences. The adult type is a slowly growing mass which typically involves the head and neck. Fetal type is seen in the head and neck region. The genital type is almost always found in the vulvovaginal region of older women. Treatment of adult type rhabdomyoma of head and neck depends upon the severity of clinical symptoms.
Classification
Rhabdomyoma may be classified into the following subtypes: [1][2][3][4][5][6]
Neoplastic
Neoplastic rhabdomyomas may be further classified into the following types:
Hamartomataous
Hamartamatous rhabdomyomas may be further classified into the following types:
- Cardiac rhabdomyoma
- Rhabdomyomatous mesenchymal hamartomas of the skin(RMH) which is a rare congenital malformation involving the dermis and subcutaneous tissue, it can present as skin tag or even trigeminal neuralgia case reported. [7]
Staging
There have not been sufficient studies for the staging of rhabdomyoma. There is staging based on site, tumor size and metastases for rhabdomyosarcoma, which is a malignant tumor of striated muscle.
Pathophysiology
Pathogenesis
Cardiac Rhabdomyoma
- Cardiac rhabdomyoma is typically seen in cases of tuberous sclerosis and the pathogenesis involves mutations in the TSC1 and TSC2 genes.[8]
- Mutations in the TSC1 and TSC2 genes affect downstream molecular signalling pathways, primarily the mTOR pathway that leads to disrupted cellular growth, proliferation and motility.[9][10]
- Activation of mTOR pathway leads to increased translation and protein production by ribosomal protein S6 kinase beta-1 (p70S6K) and eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1), contributing to the abnormal cell growth and proliferation
- Cardiac rhabdomyoma is usually diagnosed during the second or third trimester on ultrasound, rhabdomyoma appears as round, homogeneous, hyperechogenic masses in the ventricles, and they sometimes appear as multiple foci in the ventricles and septal wall. Differential diagnosis between rhabdomyoma, fibroma or myxoma using ultrasonography for a single cardiac mass remains difficult. [11][12]
- Cells usually lose their ability to divide and undergo apoptosis via a ubiquitin-mediated pathway and regression of the hamartoma ensues.[13]
- Result can be complete or partial regression of hamartoma tumor.
Extracardiac Rhabdomyoma
- The pathogenesis of extracardiac rhabdomyoma is largely unknown, however, constitutive activation of the Hedgehog signalling (SHH pathway activation) and association with Gorlin’s syndrome have been implicated as the two key mechanisms leading to development of these soft tissue tumors.[14][15]
Location
- Approximately 90% of adult rhabdomyoma are found in head and neck.[16][17]
- Adult rhabdomyoma is localized to the oropharynx, the larynx, and the muscles of the neck.
- Fetal rhabdomyoma occurs most often in the subcutaneous tissues of the head and neck in children.
- Genital rhabdomyoma most often involves the vagina or vulva.
- Location of cardiac rhabdomyoma determines the clinical presentation, it usually is multi foci and involves the myocardium of both ventricles and/or the interventricular septum, but can be found in the atrium as well as endocardium.
Immunohistochemistry
- Immunohistological cell markers include:
- Muscle specific actin, phosphotungstic acid hematoxylin (PTAH), desmin, and myoglobin.[16]
- Dystrophin is shown to be expressed in the cell membranes.
Associated Conditions
- Cardiac rhabdomyoma is associated with a variety of abnormalities such as :[18]
- Tuberous sclerosis (most common associated genetical abnormality)
- Brain lesions
- Down syndrome
- Wolff-Parkinson-White syndrome (WPW)
- Ebstein anomaly
- Tetralogy of Fallot
- Hypoplastic left heart syndrome
Gross Pathology
- On gross pathology, round or polypoid mass in the region of the neck are characteristic findings of adult rhabdomyoma.
- Round or lobulated, well-circumscribed masses which can be up to 10 cm in diameter.[19]
- Isolated or multiple.
- Solid tan-white homogeneous consistency, often watery and glistening on their cut surface.
- Infrequently, calcification and hemorrhage.

Microscopic Pathology
- On microscopic histopathological analysis, characteristic findings of adult rhabdomyoma include:
- Polygonal cells with eosinophilic granular cytoplasm that is mixed with intracellular vacuolated cells.
- Rhabdomyomas are very well differentiated similarly to striated muscle cells which present markers such as actin, desmin, and myoglobin; dystrophin on their cell membranes. [20]
- Histopathologic findings of fetal rhabdomyoma include spindle-shaped cells with vacuolated clear cytoplasm due to loss of glycogen or granulated ones.
- On microscopic histopathological analysis, characteristic findings of genital rhabdomyoma include:
- Fibroblast cells among them mature striated cells.
- A matrix containing varying amounts of collagen and mucoid material
- On microscopic histopathological analysis, characteristic findings of cardiac rhabdomyoma include of classic "spider cells" muscle cells similar to embryonic myofibrils.[21]
- On microscopic histopathological findings of Rhabdomyomatous mesenchymal hamartoma (RMH) which is a rare congenital lesion of the dermis and soft tissues consisting of a disordered and varied collection of mature adipose tissue, skeletal muscle, adnexal elements, and nerve bundles. Rhabdomyomatous mesenchymal hamartoma has different names including striated muscle hamartoma, congenital midline hamartoma, and hamartoma of cutaneous adnexa and mesenchyme. Several published cases report the occurrence of RMH within the setting of other uncommon congenital abnormalities.[20]

Causes
- Adult rhabdomyomas are matured neoplasms of clonal origin.
- Cardiac rhabdomyoma may be caused by either sporadic mutation or in the setting of certain genetic disorders.[22]
- The genetic disorder commonly associated with cardiac rhabdomyoma is tuberous sclerosis.[23] Other genetic disorders associated with cardiac rhabdomyomas include basal cell nevus syndrome and Down syndrome in the setting of tuberous sclerosis.[24][25]
- The familial form of tuberous sclerosis are hamartomas that can involve the kidneys, heart, skin, brain, and other organs.
- Cardiac rhabdomyoma can be the only presenting sign of tuberous sclerosis and there are strongly related, in fact, tuberous sclerosis in a fetus can be detected as cardiac rhabdomyoma on ultrasound.[11]
- Molecular evidence of this association has been identified as the TSC2 gene missense mutation.
- Cardiac rhabdomyoma is caused by a mutation in the TSC1 on chromosome 9q34 that encodes for protein hamartin, and TSC2 on 16p13 that encodes for tuberin. These genes are both tumor suppressor genes that assist in the regulation of growth and differentiation of developing cardiomyocytes[26]
Differentiating Rhabdomyoma from Other Diseases
- Rhabdomyomas must be differentiated from other diseases, such as:[27]
- Fibroma
- Rhabdomyosarcoma
- Hibernoma
- Reticulohistiocytoma
- Tuberous sclerosis
- Granular cell tumors
Differential Diagnosis of Cardiac Rhabdomyoma
Cardiac rhabdomyoma should be differentiated from other cardiac tumors that present as a cardiac mass. The following are the differentials:[28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][2][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73]
| Site of Tumor | Malignant Potential | Type of Tumor | Tissue of Origin | Age of Presentation | Location | Morphology | Signs and Symptoms | MRI Findings | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Systemic Manifestations | Cardiac Manifestations | Embolic Manifestations | ||||||||
| Primary Cardiac Tumor | Primary Benign | Myxoma |
|
|
|
|
|
|
|
|
| Rhabdomyoma |
|
|
|
|
|
|
| |||
| Fibroma |
|
|
|
|
|
|
| |||
| Fibroelastoma |
|
|
|
|
|
|
| |||
| Hemangioma |
|
|
|
|
|
|
|
| ||
| Lipoma |
|
|
|
|
|
|
|
| ||
| Paraganglioma |
|
|
|
|
|
|
|
| ||
| Atrioventricular Node Tumor |
|
|
|
|
|
|
|
| ||
| Lipomatous hypertrophy of the interatrial septum |
|
|
|
|
|
|
- |
| ||
| Primary Malignant | Fibrosarcoma |
|
|
|
|
|
|
| ||
| Angiosarcoma |
|
|
|
|
|
|
|
| ||
| Rhabdomyosarcoma |
|
|
|
|
|
|
|
| ||
| Lymphoma |
|
|
|
|
|
|
|
|||
| Secondary CardiacTumor | Metastastatic Malignant | Metastasis |
|
|
|
|
|
|
|
|
Epidemiology and Demographics
- Cardiac rhabdomyomas are usually detected during the first year of life or before birth and accounts for majority of all primary cardiac tumors.[24][74][75]
- Worldwide, rhabdomyoma is rare.
- Most of patients with tuberous sclerosis develop a cardiac rhabdomyoma. Similarly, children diagnosed with cardiac rhabdomyomas demonstrate radiologic or clinical findings of tuberous sclerosis or have a positive family history. Rhabdomyoma is extremely rare in the United States. Rhabdomyoma has a relative incidence of 5.8%.
Age
- Adult rhabdomyoma is more commonly observed among patients aged greater than 40 years old.[12]
- Fetal rhabdomyoma is more commonly observed among patients aged between birth and 3 years.
- Cardiac rhabdomyoma is more commonly observed among patients in the pediatric age group.
- Genital rhabdomyoma is more commonly observed among patients in the young and middle-aged women.
- Rhabdomyomatous mesenchymal hamartomas of the skin is more commonly observed among newborns and infants.[1]
Gender
- Cardiac rhabdomyoma affects men and women equally.[15][76]
- Rhabdomyomatous mesenchymal hamartoma of skin is extremely rare in both genders.
- Males are more commonly affected with adult rhabdomyoma than females.
- Males are more commonly affected with fetal rhabdomyoma than females.
- Females are more commonly affected with genital rhabdomyoma than males.
Race
- There is no racial predilection for rhabdomyomas.
Risk Factors
- There are no established risk factors for rhabdomyoma.
Natural History, Complications and Prognosis
Natural History
- The majority of cases regress spontaneously(partial or complete),however there are reported cases which surgical interventions were necessary,especially if tumor causes cardiac symptoms such as arrhythmia or obstruction. [77][3][44]
- Early clinical features include heart failure, cardiac murmur, and arrhythmia.
- Tumors larger in diameter are more likely to cause arrhythmias or hemodynamic instability, which are associated with an increased risk of death.
- The majority of patients with cardiac rhabdomyoma remain asymptomatic; however, some affected patients become symptomatic in the perinatal period.
Complications
- Common complications of cardiac rhabdomyoma include development of cardiac arrhythmias, ventricular outflow tract obstruction, and congestive heart failure.[78]
Prognosis
- prognosis is generally good; the survival rate of patients with benign rhabdomyoma is excellent, depending on location of tumor, prognosis may change.[79]
- Rhabdomyomas on mitral or tricuspid valves can lead to regurgitation or obstruction of outflow tract thus poor prognosis.[80]
- The long-term prognosis of cardiac rhabdomyoma is affected by the neurologic manifestations associated with tuberous sclerosis.[81]
- The prognosis of patients with rhabdomyomas depends mainly on the size, location, number of the lesions, associated anomalies such as tuberous sclerosis.
- Metastases have not been associated with rhabdomyoma.
Diagnosis
Symptoms
- Symptoms of adult rhabdomyoma may include:[82]
- Hoarseness
- Difficulty breathing
- Difficulty swallowing(dysphagia)
- laryngeal mass
- In a very rare occassion can present with symptoms of hyperparathyroidism if it involves parathyroid gland. [83]
- Symptoms of genital rhabdomyoma may include the following:
- Symptoms of cardiac rhabdomyoma may include the following:
- In cardiac rhabdomyoma, symptoms if present, may be caused by obstruction of blood flow through the heart or consist of rhythm disturbances, such as heart block and ventricular tachycardia.[84][45]
Physical Examination
- Physical examination may be remarkable for:
- The presence of a round or polypoid mass in the region of the neck in adult rhabdomyoma
- Subcutaneous masses in the head and neck regions in fetal rhabdomyoma
- Vaginal masses in genital rhabdomyoma
- Cardiac rhabdomyoma may present with mitral or tricuspid regurgitation murmur.[85]
Laboratory Findings
- There are no specific laboratory findings associated with rhabdomyoma.
- The following laboratory studies may be ordered to rule out additional conditions:
Imaging Findings
- MRI is the imaging modality of choice for rhabdomyoma. Chest CT scan may be helpful in the diagnosis of cardiac rhabdomyoma.
- On ultrasound, rhabdomyoma is identified by single or multi foci hyper echoic mass(es) located adjacent to the myocardium.[28]
- X-Rays of the chest and affected areas of the body may be helpful in the diagnosis of rhabdomyomas.
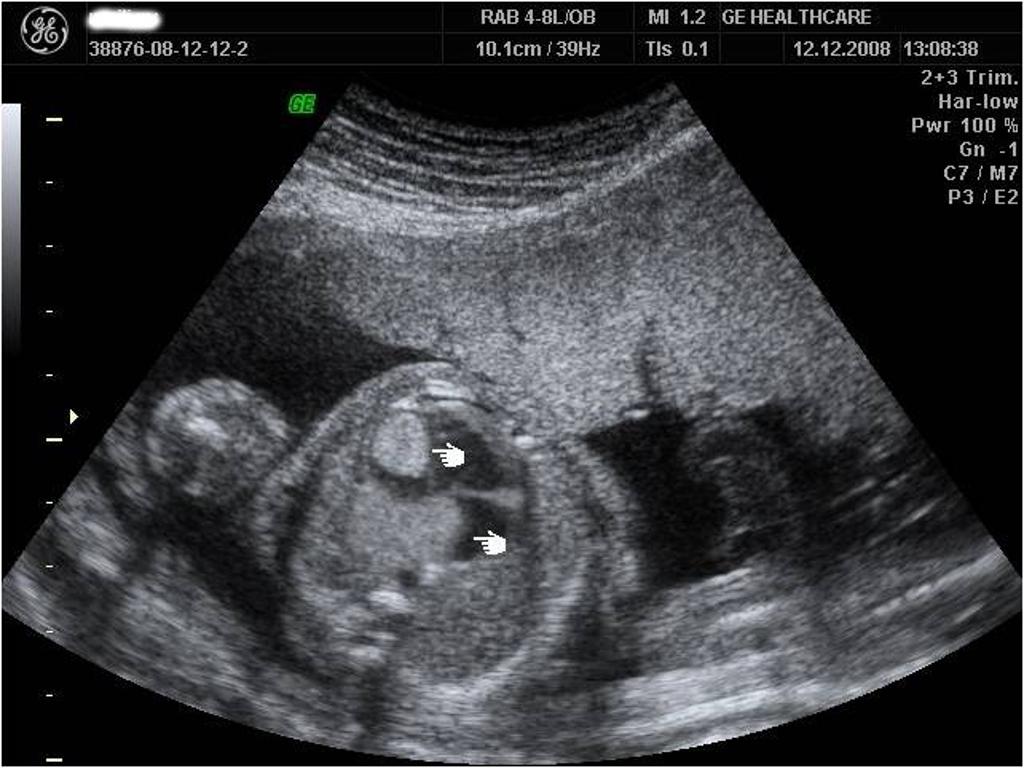
Other Diagnostic Studies
- Rhabdomyoma may also be diagnosed using biopsy since overlap in morphologic features between rhabdomyosarcoma (RMS) vs. rhabdomyoma makes differential diagnosis difficult.[86]
- Any masses, including those found in the head and neck of patients with adult rhabdomyoma, should be biopsied to establish a diagnosis.
Treatment
Medical Therapy
- Treatment for rhabdomyoma is supportive care.[87][88] [89]
- patients with rhabdomyoma should be monitored with echocardiography and electrocardiography.
Adult rhabdomyoma
- Patients with laryngeal rhabdomyoma need immediate care such as nasal oxygen, intravenous fluids and if respiratory distress develops intubation before admission for surgery and surgical excision. [17]
- Patients with adult rhabdomyoma and shortness of breath should restrain from activities which exacerbate their breathing difficulty.
Cardiac rhabdomyoma
- Pharmacological treatment available for fetal cardiac rhabdomyoma is everolimus.[90]
- Patients with arrhythmias are treated with antiarrhythmic medications.
- Restriction on physical activities in those patients with cardiac clinical symptoms.
- Asymptomatic children with TSC and a rhabdomyoma should have echocardiography every one to three years until regression of cardiac rhabdomyoma is documented.
Genital rhabdomyoma
- In patients with genital rhabdomyoma, urinary tract obstruction may happen, therefore catheterization use may be necessary in order to avoid urinary tract infection.
- Pregnant patients need to be monitored closely, as they may require a cesarean delivery.[6][91]
Surgery
Adult rhabdomyoma
- Surgical resection of the tumor can only be performed for patients with adult rhabdomyoma if airway obstruction is diagnosed, there have been reports of rare cases of laryngeal rhabdomyoma which may cause breathing difficulty for patients.[92]
Cardiac rhabdomyoma
- In patients with cardiac rhabdomyoma who have symptoms of severe outflow tract obstruction or arrhythmias, surgical intervention can be helpful. Surgical management involves removal of the part of the tumor causing obstruction without complete excision of the entire lesion.[93]
Prevention
- There are no primary preventive measures available for rhabdomyoma.
References
- ↑ 1.0 1.1 McKinnon EL, Rand AJ, Selim MA, Fuchs HE, Buckley AF, Cummings TJ (October 2015). "Rhabdomyomatous mesenchymal hamartoma presenting as a sacral skin tag in two neonates with spinal dysraphism". J. Cutan. Pathol. 42 (10): 774–8. doi:10.1111/cup.12538. PMID 25989364.
- ↑ 2.0 2.1 Beghetti M, Gow RM, Haney I, Mawson J, Williams WG, Freedom RM (1997). "Pediatric primary benign cardiac tumors: a 15-year review". Am Heart J. 134 (6): 1107–14. PMID 9424072.
- ↑ 3.0 3.1 Becker AE (2000). "Primary heart tumors in the pediatric age group: a review of salient pathologic features relevant for clinicians". Pediatr Cardiol. 21 (4): 317–23. doi:10.1007/s002460010071. PMID 10865004.
- ↑ Elderkin RA, Radford DJ (2002). "Primary cardiac tumours in a paediatric population". J Paediatr Child Health. 38 (2): 173–7. PMID 12031001.
- ↑ Kocabaş A, Ekici F, Cetin Iİ, Emir S, Demir HA, Arı ME; et al. (2013). "Cardiac rhabdomyomas associated with tuberous sclerosis complex in 11 children: presentation to outcome". Pediatr Hematol Oncol. 30 (2): 71–9. doi:10.3109/08880018.2012.734896. PMID 23151153.
- ↑ 6.0 6.1 Lu DY, Chang S, Cook H, Alizadeh Y, Karam AK, Moatamed NA, Dry SM (April 2012). "Genital rhabdomyoma of the urethra in an infant girl". Hum. Pathol. 43 (4): 597–600. doi:10.1016/j.humpath.2011.06.012. PMID 21992817.
- ↑ White LR, Agrawal V, Sutton L, Balbosa AC (June 2015). "Rhabdomyomatous mesenchymal hamartoma of the face causing trigeminal neuralgia". Am J Case Rep. 16: 338–40. doi:10.12659/AJCR.893719. PMC 4460909. PMID 26037964.
- ↑ "Enzinger and Weiss's Soft Tissue Tumors - 6th Edition".
- ↑ Kotulska K, Larysz-Brysz M, Grajkowska W, Jóźwiak J, Włodarski P, Sahin M, Lewin-Kowalik J, Domańska-Pakieła D, Jóźwiak S (2009). "Cardiac rhabdomyomas in tuberous sclerosis complex show apoptosis regulation and mTOR pathway abnormalities". Pediatr. Dev. Pathol. 12 (2): 89–95. doi:10.2350/06-11-0191.1. PMID 17990907.
- ↑ Krueger DA, Northrup H (October 2013). "Tuberous sclerosis complex surveillance and management: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference". Pediatr. Neurol. 49 (4): 255–65. doi:10.1016/j.pediatrneurol.2013.08.002. PMID 24053983.
- ↑ 11.0 11.1 Sharma N, Sharma S, Thiek JL, Ahanthem SS, Kalita A, Lynser D (2017). "Maternal and Fetal Tuberous Sclerosis: Do We Know Enough as an Obstetrician?". J Reprod Infertil. 18 (2 pages=257–260). PMC 5565905. PMID 28868251.
- ↑ 12.0 12.1 Bejiqi R, Retkoceri R, Bejiqi H (April 2017). "Prenatally Diagnosis and Outcome of Fetuses with Cardiac Rhabdomyoma - Single Centre Experience". Open Access Maced J Med Sci. 5 (2): 193–196. doi:10.3889/oamjms.2017.040. PMC 5420773. PMID 28507627.
- ↑ Wu SS, Collins MH, de Chadarévian JP (2002). "Study of the regression process in cardiac rhabdomyomas". Pediatr. Dev. Pathol. 5 (1): 29–36. PMID 11815866.
- ↑ Hettmer S, Teot LA, Kozakewich H, Werger AM, Davies KJ, Fletcher CD, Grier HE, Rodriguez-Galindo C, Wagers AJ (March 2015). "Myogenic tumors in nevoid Basal cell carcinoma syndrome". J. Pediatr. Hematol. Oncol. 37 (2): 147–9. doi:10.1097/MPH.0000000000000115. PMC 4127382. PMID 24517962.
- ↑ 15.0 15.1 de Trey LA, Schmid S, Huber GF (2013). "Multifocal adult rhabdomyoma of the head and neck manifestation in 7 locations and review of the literature". Case Rep Otolaryngol. 2013: 758416. doi:10.1155/2013/758416. PMC 3697226. PMID 23841004.
- ↑ 16.0 16.1 Bjørndal Sørensen K, Godballe C, Ostergaard B, Krogdahl A (March 2006). "Adult extracardiac rhabdomyoma: light and immunohistochemical studies of two cases in the parapharyngeal space". Head Neck. 28 (3): 275–9. doi:10.1002/hed.20358. PMID 16419079.
- ↑ 17.0 17.1 Amelia Souza A, de Araújo VC, Passador Santos F, Ferreira Martinez E, de Menezes Filho JF, Soares de Araujo N, Soares AB (2013). "Intraoral adult rhabdomyoma: a case report". Case Rep Dent. 2013: 741548. doi:10.1155/2013/741548. PMC 3833031. PMID 24288631.
- ↑ Castilla Cabanes E, Lacambra Blasco I (2014). "Multiple cardiac rhabdomyomas, wolff-Parkinson-white syndrome, and tuberous sclerosis: an infrequent combination". Case Rep Pediatr. 2014: 973040. doi:10.1155/2014/973040. PMC 4189844. PMID 25328743.
- ↑ Kassop D, Donovan MS, Cheezum MK, Nguyen BT, Gambill NB, Blankstein R, Villines TC (2014). "Cardiac Masses on Cardiac CT: A Review". Curr Cardiovasc Imaging Rep. 7: 9281. doi:10.1007/s12410-014-9281-1. PMC 4090749. PMID 25018846.
- ↑ 20.0 20.1 Rosenberg AS, Kirk J, Morgan MB (April 2002). "Rhabdomyomatous mesenchymal hamartoma: an unusual dermal entity with a report of two cases and a review of the literature". J. Cutan. Pathol. 29 (4): 238–43. PMID 12028157.
- ↑ Fenoglio JJ, MCAllister HA, Ferrans VJ (August 1976). "Cardiac rhabdomyoma: a clinicopathologic and electron microscopic study". Am. J. Cardiol. 38 (2): 241–51. PMID 952267.
- ↑ Burke A, Virmani R (2008). "Pediatric heart tumors". Cardiovasc Pathol. 17 (4): 193–8. doi:10.1016/j.carpath.2007.08.008. PMID 18402818.
- ↑ Vaughan CJ, Veugelers M, Basson CT (2001). "Tumors and the heart: molecular genetic advances". Curr Opin Cardiol. 16 (3): 195–200. PMID 11357016.
- ↑ 24.0 24.1 Isaacs H (2004). "Fetal and neonatal cardiac tumors". Pediatr Cardiol. 25 (3): 252–73. doi:10.1007/s00246-003-0590-4. PMID 15360117.
- ↑ Krapp M, Baschat AA, Gembruch U, Gloeckner K, Schwinger E, Reusche E (1999). "Tuberous sclerosis with intracardiac rhabdomyoma in a fetus with trisomy 21: case report and review of literature". Prenat Diagn. 19 (7): 610–3. PMID 10419607.
- ↑ Goldenberg DL (November 1989). "A review of the role of tricyclic medications in the treatment of fibromyalgia syndrome". J Rheumatol Suppl. 19: 137–9. PMID 2691673.
- ↑ Nasr E, Ibrahim M, Yacoub M (January 2017). "Heart failure in a neonate with multiple cardiac masses". Heart. 103 (1): 18. doi:10.1136/heartjnl-2016-310251. PMID 27655257.
- ↑ 28.0 28.1 Mankad R, Herrmann J (December 2016). "Cardiac tumors: echo assessment". Echo Res Pract. 3 (4): R65–R77. doi:10.1530/ERP-16-0035. PMC 5292983. PMID 27600455.
- ↑ Zaragoza-Macias E, Zaragosa-Macias E, Chen MA, Gill EA (February 2012). "Real time three-dimensional echocardiography evaluation of intracardiac masses". Echocardiography. 29 (2): 207–19. doi:10.1111/j.1540-8175.2011.01627.x. PMID 22283202.
- ↑ Larrieu AJ, Jamieson WR, Tyers GF, Burr LH, Munro AI, Miyagishima RT, Gerein AN, Allen P (March 1982). "Primary cardiac tumors: experience with 25 cases". J. Thorac. Cardiovasc. Surg. 83 (3): 339–48. PMID 7062746.
- ↑ Molina JE, Edwards JE, Ward HB (August 1990). "Primary cardiac tumors: experience at the University of Minnesota". Thorac Cardiovasc Surg. 38 Suppl 2: 183–91. doi:10.1055/s-2007-1014064. PMID 2237900.
- ↑ Tazelaar HD, Locke TJ, McGregor CG (October 1992). "Pathology of surgically excised primary cardiac tumors". Mayo Clin. Proc. 67 (10): 957–65. PMID 1434856.
- ↑ Sarjeant JM, Butany J, Cusimano RJ (2003). "Cancer of the heart: epidemiology and management of primary tumors and metastases". Am J Cardiovasc Drugs. 3 (6): 407–21. doi:10.2165/00129784-200303060-00004. PMID 14728061.
- ↑ St John Sutton MG, Mercier LA, Giuliani ER, Lie JT (June 1980). "Atrial myxomas: a review of clinical experience in 40 patients". Mayo Clin. Proc. 55 (6): 371–6. PMID 7382545.
- ↑ Pinede L, Duhaut P, Loire R (May 2001). "Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases". Medicine (Baltimore). 80 (3): 159–72. PMID 11388092.
- ↑ Reynen K (December 1995). "Cardiac myxomas". N. Engl. J. Med. 333 (24): 1610–7. doi:10.1056/NEJM199512143332407. PMID 7477198.
- ↑ Javed A, Zalawadiya S, Kovach J, Afonso L (March 2014). "Aortic valve myxoma at the extreme age: a review of literature". BMJ Case Rep. 2014. doi:10.1136/bcr-2013-202689. PMC 3962858. PMID 24642215.
- ↑ Lee VH, Connolly HM, Brown RD (August 2007). "Central nervous system manifestations of cardiac myxoma". Arch. Neurol. 64 (8): 1115–20. doi:10.1001/archneur.64.8.1115. PMID 17698701.
- ↑ Carney JA, Gordon H, Carpenter PC, Shenoy BV, Go VL (July 1985). "The complex of myxomas, spotty pigmentation, and endocrine overactivity". Medicine (Baltimore). 64 (4): 270–83. PMID 4010501.
- ↑ McAllister HA, Hall RJ, Cooley DA (February 1999). "Tumors of the heart and pericardium". Curr Probl Cardiol. 24 (2): 57–116. PMID 10028128.
- ↑ Klarich KW, Enriquez-Sarano M, Gura GM, Edwards WD, Tajik AJ, Seward JB (September 1997). "Papillary fibroelastoma: echocardiographic characteristics for diagnosis and pathologic correlation". J. Am. Coll. Cardiol. 30 (3): 784–90. PMID 9283541.
- ↑ Tamin SS, Maleszewski JJ, Scott CG, Khan SK, Edwards WD, Bruce CJ, Oh JK, Pellikka PA, Klarich KW (June 2015). "Prognostic and Bioepidemiologic Implications of Papillary Fibroelastomas". J. Am. Coll. Cardiol. 65 (22): 2420–9. doi:10.1016/j.jacc.2015.03.569. PMID 26046736.
- ↑ Gowda RM, Khan IA, Nair CK, Mehta NJ, Vasavada BC, Sacchi TJ (September 2003). "Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases". Am. Heart J. 146 (3): 404–10. doi:10.1016/S0002-8703(03)00249-7. PMID 12947356.
- ↑ 44.0 44.1 Smythe JF, Dyck JD, Smallhorn JF, Freedom RM (November 1990). "Natural history of cardiac rhabdomyoma in infancy and childhood". Am. J. Cardiol. 66 (17): 1247–9. PMID 2239731.
- ↑ 45.0 45.1 Jacobs JP, Konstantakos AK, Holland FW, Herskowitz K, Ferrer PL, Perryman RA (November 1994). "Surgical treatment for cardiac rhabdomyomas in children". Ann. Thorac. Surg. 58 (5): 1552–5. PMID 7979700.
- ↑ Elbardissi AW, Dearani JA, Daly RC, Mullany CJ, Orszulak TA, Puga FJ, Schaff HV (September 2008). "Survival after resection of primary cardiac tumors: a 48-year experience". Circulation. 118 (14 Suppl): S7–15. doi:10.1161/CIRCULATIONAHA.107.783126. PMID 18824772.
- ↑ Basu S, Folliguet T, Anselmo M, Greengart A, Sabado M, Cunningham JN, Jacobowitz IJ (April 1994). "Lipomatous hypertrophy of the interatrial septum". Cardiovasc Surg. 2 (2): 229–31. PMID 8049952.
- ↑ Simpson L, Kumar SK, Okuno SH, Schaff HV, Porrata LF, Buckner JC, Moynihan TJ (June 2008). "Malignant primary cardiac tumors: review of a single institution experience". Cancer. 112 (11): 2440–6. doi:10.1002/cncr.23459. PMID 18428209.
- ↑ Vander Salm TJ (April 2000). "Unusual primary tumors of the heart". Semin. Thorac. Cardiovasc. Surg. 12 (2): 89–100. PMID 10807431.
- ↑ Petersen CD, Robinson WA, Kurnick JE (1976). "Involvement of the heart and pericardium in the malignant lymphomas". Am. J. Med. Sci. 272 (2): 161–5. PMID 1008078.
- ↑ Ragland MM, Tak T (March 2006). "The role of echocardiography in diagnosing space-occupying lesions of the heart". Clin Med Res. 4 (1): 22–32. PMC 1447535. PMID 16595790.
- ↑ Miguel CE, Bestetti RB (June 2011). "Primary cardiac lymphoma". Int. J. Cardiol. 149 (3): 358–63. doi:10.1016/j.ijcard.2010.02.016. PMID 20227122.
- ↑ Grebenc ML, Rosado de Christenson ML, Burke AP, Green CE, Galvin JR (2000). "Primary cardiac and pericardial neoplasms: radiologic-pathologic correlation". Radiographics. 20 (4): 1073–103, quiz 1110–1, 1112. doi:10.1148/radiographics.20.4.g00jl081073. PMID 10903697.
- ↑ Grinda JM, Couetil JP, Chauvaud S, D'Attellis N, Berrebi A, Fabiani JN, Deloche A, Carpentier A (January 1999). "Cardiac valve papillary fibroelastoma: surgical excision for revealed or potential embolization". J. Thorac. Cardiovasc. Surg. 117 (1): 106–10. PMID 9869763.
- ↑ Webb DW, Thomas RD, Osborne JP (March 1993). "Cardiac rhabdomyomas and their association with tuberous sclerosis". Arch. Dis. Child. 68 (3): 367–70. PMC 1793857. PMID 8466239.
- ↑ Yoshitake I, Hata M, Sezai A, Niino T, Unosawa S, Shimura K, Kasamaki Y, Minami K (September 2009). "Cardiac angiosarcoma with cardiac tamponade diagnosed as a ruptured aneurysm of the sinus valsalva". Jpn. J. Clin. Oncol. 39 (9): 612–5. doi:10.1093/jjco/hyp044. PMID 19493870.
- ↑ Parissis H, Akbar MT, Young V (October 2010). "Primary leiomyosarcoma of the right atrium: a case report and literature update". J Cardiothorac Surg. 5: 80. doi:10.1186/1749-8090-5-80. PMC 2964688. PMID 20939891.
- ↑ Gulati G, Sharma S, Kothari SS, Juneja R, Saxena A, Talwar KK (2004). "Comparison of echo and MRI in the imaging evaluation of intracardiac masses". Cardiovasc Intervent Radiol. 27 (5): 459–69. doi:10.1007/s00270-004-0123-4. PMID 15383848.
- ↑ Narin B, Arman A, Arslan D, Simşek M, Narin A (February 2010). "Assessment of cardiac masses: magnetic resonance imaging versus transthoracic echocardiography". Anadolu Kardiyol Derg. 10 (1): 69–74. PMID 20150010.
- ↑ "academic.oup.com".
- ↑ Ismail I, Al-Khafaji K, Mutyala M, Aggarwal S, Cotter W, Hakim H, Khosla S, Arora R (2015). "Cardiac lipoma". J Community Hosp Intern Med Perspect. 5 (5): 28449. doi:10.3402/jchimp.v5.28449. PMC 4612478. PMID 26486106.
- ↑ D'Souza J, Shah R, Abbass A, Burt JR, Goud A, Dahagam C (January 2017). "Invasive Cardiac Lipoma: a case report and review of literature". BMC Cardiovasc Disord. 17 (1): 28. doi:10.1186/s12872-016-0465-2. PMC 5237479. PMID 28088193.
- ↑ Yadav, Pradeep K.; Baquero, Giselle A.; Malysz, Jozef; Kelleman, John; Gilchrist, Ian C. (2014). "Cardiac Paraganglioma". Circulation: Cardiovascular Interventions. 7 (6): 851–856. doi:10.1161/CIRCINTERVENTIONS.114.001856. ISSN 1941-7640.
- ↑ Tahir M, Noor SJ, Herle A, Downing S (2009). "Right atrial paraganglioma: a rare primary cardiac neoplasm as a cause of chest pain". Tex Heart Inst J. 36 (6): 594–7. PMC 2801953. PMID 20069088.
- ↑ Hamilton BH, Francis IR, Gross BH, Korobkin M, Shapiro B, Shulkin BL, Deeb CM, Orringer MB (January 1997). "Intrapericardial paragangliomas (pheochromocytomas): imaging features". AJR Am J Roentgenol. 168 (1): 109–13. doi:10.2214/ajr.168.1.8976931. PMID 8976931.
- ↑ Shih, Wei-Jen; McCullough, Scott; Smith, Mary (1993). "Diagnostic imagings for primary cardiac fibrosarcoma". International Journal of Cardiology. 39 (2): 157–161. doi:10.1016/0167-5273(93)90028-F. ISSN 0167-5273.
- ↑ Arai T, Kurashima C, Wada S, Chida K, Ohkawa S (November 1998). "Histological evidence for cell proliferation activity in cystic tumor (endodermal heterotopia) of the atrioventricular node". Pathol. Int. 48 (11): 917–23. PMID 9832064.
- ↑ Wolf PL, Bing R (November 1965). "The smallest tumor which causes sudden death". JAMA. 194 (6): 674–5. PMID 5897246.
- ↑ Burke AP, Anderson PG, Virmani R, James TN, Herrera GA, Ceballos R (October 1990). "Tumor of the atrioventricular nodal region. A clinical and immunohistochemical study". Arch. Pathol. Lab. Med. 114 (10): 1057–62. PMID 2222148.
- ↑ Burke A, Tavora F (April 2016). "The 2015 WHO Classification of Tumors of the Heart and Pericardium". J Thorac Oncol. 11 (4): 441–52. doi:10.1016/j.jtho.2015.11.009. PMID 26725181.
- ↑ Tran, Thao T; Starnes, Vaughn; Wang, Xuedong; Getzen, James; Ross, Brian D (2009). "Cardiovascular magnetics resonance diagnosis of cystic tumor of the atrioventricular node". Journal of Cardiovascular Magnetic Resonance. 11 (1): 13. doi:10.1186/1532-429X-11-13. ISSN 1532-429X.
- ↑ Tatli, Servet; O'Gara, Patrick Thomas; Lambert, Jarvis; Kwong, Raymond; Byrne, John Gerald; Yucel, E. Kent (2004). "MRI of Atypical Lipomatous Hypertrophy of the Interatrial Septum". American Journal of Roentgenology. 182 (3): 598–600. doi:10.2214/ajr.182.3.1820598. ISSN 0361-803X.
- ↑ Saboo, Sachin S.; Krajewski, Katherine M.; Zukotynski, Katherine; Howard, Stephanie; Jagannathan, Jyothi P.; Hornick, Jason L.; Ramaiya, Nikhil (2012). "Imaging Features of Primary and Secondary Adult Rhabdomyosarcoma". American Journal of Roentgenology. 199 (6): W694–W703. doi:10.2214/AJR.11.8213. ISSN 0361-803X.
- ↑ Delides A, Petrides N, Banis K (2005). "Multifocal adult rhabdomyoma of the head and neck: a case report and literature review". Eur Arch Otorhinolaryngol. 262 (6): 504–6. doi:10.1007/s00405-004-0840-y. PMID 15942804.
- ↑ Ramadani N, Kreshnike KD, Muçaj S, Kabashi S, Hoxhaj A, Jerliu N, Bejiçi R (April 2016). "MRI Verification of a Case of Huge Infantile Rhabdomyoma". Acta Inform Med. 24 (2): 146–8. doi:10.5455/aim.2016.24.146-148. PMC 4851540. PMID 27147810.
- ↑ Takeyama J, Hayashi T, Sanada T, Shimanuki Y, Saito M, Shirane R (April 2005). "Rhabdomyomatous mesenchymal hamartoma associated with nasofrontal meningocele and dermoid cyst". J. Cutan. Pathol. 32 (4): 310–3. doi:10.1111/j.0303-6987.2005.00312.x. PMID 15769282.
- ↑ Barnes BT, Procaccini D, Crino J, Blakemore K, Sekar P, Sagaser KG, Jelin AC, Gaur L (May 2018). "Maternal Sirolimus Therapy for Fetal Cardiac Rhabdomyomas". N. Engl. J. Med. 378 (19): 1844–1845. doi:10.1056/NEJMc1800352. PMC 6201692. PMID 29742370.
- ↑ Hinton RB, Prakash A, Romp RL, Krueger DA, Knilans TK (November 2014). "Cardiovascular manifestations of tuberous sclerosis complex and summary of the revised diagnostic criteria and surveillance and management recommendations from the International Tuberous Sclerosis Consensus Group". J Am Heart Assoc. 3 (6): e001493. doi:10.1161/JAHA.114.001493. PMC 4338742. PMID 25424575.
- ↑ Linnemeier L, Benneyworth BD, Turrentine M, Rodefeld M, Brown J (April 2015). "Pediatric cardiac tumors: a 45-year, single-institution review". World J Pediatr Congenit Heart Surg. 6 (2): 215–9. doi:10.1177/2150135114563938. PMID 25870340.
- ↑ Wang Y, Wang X, Xiao Y (February 2016). "Surgical treatment of primary cardiac valve tumor: early and late results in eight patients". J Cardiothorac Surg. 11: 31. doi:10.1186/s13019-016-0406-2. PMC 4759914. PMID 26891966.
- ↑ Chung C, Lawson JA, Sarkozy V, Riney K, Wargon O, Shand AW, Cooper S, King H, Kennedy SE, Mowat D (November 2017). "Early Detection of Tuberous Sclerosis Complex: An Opportunity for Improved Neurodevelopmental Outcome". Pediatr. Neurol. 76: 20–26. doi:10.1016/j.pediatrneurol.2017.05.014. PMID 28811058. Vancouver style error: initials (help)
- ↑ Altissimi G, Ralli M, Sementilli G, Fiorentino F, Ciofalo A, Greco A, de Vincentiis M, Corsi A, Cianfrone G (2017). "Adult-Type Rhabdomyoma of the Larynx: Clinicopathologic Study of an Uncommon Tumor in a Rare Location". Case Rep Otolaryngol. 2017: 7186768. doi:10.1155/2017/7186768. PMC 5727691. PMID 29318074.
- ↑ Lee JP, Blake Sullivan C, Bayon R, Shearer AE, Robinson RA (December 2018). "Adult type rhabdomyoma presenting as a parathyroid adenoma". Head Neck. doi:10.1002/hed.25419. PMID 30537102.
- ↑ Bosi G, Lintermans JP, Pellegrino PA, Svaluto-Moreolo G, Vliers A (1996). "The natural history of cardiac rhabdomyoma with and without tuberous sclerosis". Acta Paediatr. 85 (8): 928–31. PMID 8863873.
- ↑ Ono M, Boethig D, Akin E, Goerler H, Breymann T (March 2007). "Coexistent cardiac rhabdomyoma with mitral valve anomaly in patients with tuberous sclerosis: a case report". Thorac Cardiovasc Surg. 55 (2): 120–1. doi:10.1055/s-2006-924243. PMID 17377866.</ref <nowiki><nowiki>*</nowiki></nowiki>It can present with [[seizures]] or [[cerebral palsy]] if [[tuberous sclerosis]] is involved.<ref name="pmid21173003">Staley BA, Vail EA, Thiele EA (January 2011). "Tuberous sclerosis complex: diagnostic challenges, presenting symptoms, and commonly missed signs". Pediatrics. 127 (1): e117–25. doi:10.1542/peds.2010-0192. PMC 3010088. PMID 21173003.
- ↑ Zhang N, Zeng Z, Li S, Wang F, Huang P (August 2018). "High expression of EZH2 as a marker for the differential diagnosis of malignant and benign myogenic tumors". Sci Rep. 8 (1): 12331. doi:10.1038/s41598-018-30648-7. PMC 6098067. PMID 30120321.
- ↑ Tiberio D, Franz DN, Phillips JR (2011). "Regression of a cardiac rhabdomyoma in a patient receiving everolimus". Pediatrics. 127 (5): e1335–7. doi:10.1542/peds.2010-2910. PMID 21464184.
- ↑ Wagner R, Riede FT, Seki H, Hornemann F, Syrbe S, Daehnert I; et al. (2015). "Oral Everolimus for Treatment of a Giant Left Ventricular Rhabdomyoma in a Neonate-Rapid Tumor Regression Documented by Real Time 3D Echocardiography". Echocardiography. 32 (12): 1876–9. doi:10.1111/echo.13015. PMID 26199144.
- ↑ Öztunç F, Atik SU, Güneş AO (October 2015). "Everolimus treatment of a newborn with rhabdomyoma causing severe arrhythmia". Cardiol Young. 25 (7): 1411–4. doi:10.1017/S1047951114002261. PMID 26339757.
- ↑ Martínez-García A, Michel-Macías C, Cordero-González G, Escamilla-Sánchez KI, Aguinaga-Ríos M, Coronado-Zarco A, Cardona-Pérez JA (July 2018). "Giant left ventricular rhabdomyoma treated successfully with everolimus: case report and review of literature". Cardiol Young. 28 (7): 903–909. doi:10.1017/S1047951118000598. PMID 29759095.
- ↑ Dodat H, Paulhac JB, Macabeo V, Bouvier R (1987). "[Benign tumors of the posterior urethra in children. Apropos of an unusual case of rhabdomyoma of fetal type]". J Urol (Paris) (in French). 93 (1): 43–6. PMID 3031168.
- ↑ Pinho MM, de Carvalho E Castro J, Ramos RG (October 2013). "Adult rhabdomyoma of the larynx". Int Arch Otorhinolaryngol. 17 (4): 415–8. doi:10.1055/s-0033-1351671. PMC 4399195. PMID 25992049. Vancouver style error: missing comma (help)
- ↑ Norawat R, Sarkar D, Maybauer MO (2018). "Perioperative management of critical right ventricular inflow obstruction from right atrial rhabdomyoma". Ann Card Anaesth. 21 (4): 430–432. doi:10.4103/aca.ACA_233_17. PMC 6206783. PMID 30333341.