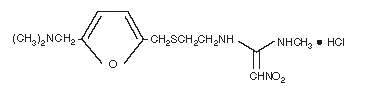Ranitidine (oral): Difference between revisions
No edit summary |
m (Aparna Vuppala moved page Ranitidine to Ranitidine (oral)) |
||
| (29 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag= | |authorTag={{Ammu}} | ||
|genericName=Ranitidine | |||
|aOrAn=a | |||
|drugClass=[[H2 receptor blocker]] | |||
|indicationType=treatment | |||
|genericName= | |indication=relieves [[heartburn]] associated with acid indigestion and sour [[stomach]] | ||
prevents [[heartburn]] associated with acid indigestion and sour [[stomach]] brought on by certain foods and beverages | |||
|adverseReactions=[[headache]], [[constipation]], [[diarrhea]], [[abdominal pain]] | |||
|blackBoxWarningTitle=Title | |||
|aOrAn= | |blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | ||
a | |||
|drugClass= | |||
| | |||
| | |||
|adverseReactions= | |||
|blackBoxWarningTitle= | |||
Title | |||
|blackBoxWarningBody= | |||
<i><span style="color:#FF0000;">ConditionName: </span></i> | |||
* Content | * Content | ||
| Line 45: | Line 16: | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult=* Short-term treatment of active [[duodenal ulcer]]. Most patients heal within 4 weeks. | |||
|fdaLIADAdult= | * Studies available to date have not assessed the safety of ranitidine in uncomplicated duodenal ulcer for periods of more than 8 weeks. | ||
* Maintenance therapy for [[duodenal ulcer]] patients at reduced dosage after healing of acute ulcers. No placebo-controlled comparative studies have been carried out for periods of longer than 1 year. | |||
* The treatment of pathological hypersecretory conditions (e.g., [[Zollinger-Ellison syndrome]] and [[systemic mastocytosis]]). | |||
* Short-term treatment of active, benign gastric ulcer. Most patients heal within 6 weeks and the usefulness of further treatment has not been demonstrated. Studies available to date have not assessed the safety of ranitidine in uncomplicated, benign gastric ulcer for periods of more than 6 weeks. | |||
* | * Maintenance therapy for gastric ulcer patients at reduced dosage after healing of acute ulcers. Placebo-controlled studies have been carried out for 1 year. | ||
* Treatment of GERD. Symptomatic relief commonly occurs within 24 hours after starting therapy with ZANTAC 150 mg twice daily. | |||
* Treatment of endoscopically diagnosed erosive esophagitis. Symptomatic relief of heartburn commonly occurs within 24 hours of therapy initiation with ZANTAC 150 mg 4 times daily. | |||
* Maintenance of healing of [[erosive esophagitis]]. Placebo-controlled trials have been carried out for 48 weeks. | |||
* Concomitant antacids should be given as needed for pain relief to patients with active duodenal ulcer; active, benign gastric ulcer; hypersecretory states; [[GERD]]; and [[erosive esophagitis]]. | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Ranitidine in adult patients. | |||
* | |offLabelAdultNoGuideSupport=* Aspiration pneumonitis; | ||
* [[Asthma]]. | |||
* Duodenitis | |||
* Gastritis medicamentosa | |||
* Hyperchlorhydria, Nocturnal. | |||
* Stress ulcer; Prophylaxis. | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
* | |||
* | |||
* | |||
* | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of | |||
|offLabelAdultNoGuideSupport= | |||
* | |||
* | |||
|offLabelPedGuideSupport= | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Non–Guideline-Supported Use (Pediatric)--> | <!--Non–Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
|offLabelPedNoGuideSupport= | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | <!--Contraindications--> | ||
|contraindications=* Ranitidine is contraindicated for patients known to have hypersensitivity to the drug or any of the ingredients. | |||
|contraindications= | |warnings======Allergy alert===== | ||
* Do not use if you are allergic to ranitidine or other acid reducers | |||
* | * Do not use if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. | ||
* With other acid reducers | |||
* If you have [[kidney]] disease, except under the advice and supervision of a doctor | |||
* Ask a doctor before use if you have | |||
:* Frequent [[chest pain]] | |||
:* Frequent [[wheezing]], particularly with heartburn unexplained weight loss | |||
:* [[Nausea]] or [[vomiting]] | |||
:* Stomach pain had heartburn over 3 months. This may be a sign of a more serious condition. | |||
:* Heartburn with lightheadedness, sweating, or dizziness chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness | |||
* Stop use and ask a doctor if your heartburn continues or worsens you need to take this product for more than 14 days | |||
* If pregnant or breast-feeding, ask a health professional before use. | |||
* Keep out of reach of children. | |||
|FDAPregCat=B | |||
|useInPregnancyFDA=* '''Pregnancy Category''' | |||
|AUSPregCat=B1 | |||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
===== | |||
| | |||
|useInPregnancyFDA= | |||
* '''Pregnancy Category''' | |||
|useInPregnancyAUS= | |||
* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | ||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |||
|useInLaborDelivery= | |useInNursing=Infant risk cannot be ruled out. | ||
There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |useInPed=There is no FDA guidance on the use of {{PAGENAME}} with respect to pediatric patients. | ||
|useInGeri=There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | |||
|useInNursing= | |useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | ||
|useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |||
|useInPed= | |useInHepaticImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | ||
There is no FDA guidance on the use of {{PAGENAME}} with respect to pediatric patients. | |useInReproPotential=There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | ||
|useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
|useInGeri= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | |||
|useInGender= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |||
|useInRace= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |||
|useInHepaticImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | |||
|useInReproPotential= | |||
There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInImmunocomp= | |||
There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration=* [[Oral]] | |||
|monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |||
<!--IV Compatibility--> | <!--IV Compatibility--> | ||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
|IVCompat= | |||
There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
<!--Overdosage--> | <!--Overdosage--> | ||
|drugBox={{Drugbox2 | |||
| Verifiedfields = changed | |||
| Watchedfields = changed | |||
| verifiedrevid = 458460406 | |||
| IUPAC_name = ''N''-(2-[(5-[(dimethylamino)methyl]furan-2-yl)methylthio]ethyl)-''N'''-methyl-2-nitroethene-1,1-diamine; dimethyl [(5-{[(2-{[1-(methylamino)-2-nitroethenyl]amino}ethyl)sulfanyl]methyl}furan-2-yl)methyl]amine | |||
| image = Ranitidine Structural Formulae.png | |||
| width = 200 | |||
| image2 = Ranitidine-A-3D-balls.png | |||
| | <!--Clinical data--> | ||
| tradename = Zantac | |||
| Drugs.com = {{drugs.com|monograph|ranitidine-hydrochloride}} | |||
| MedlinePlus = a601106 | |||
| licence_US = Ranitidine | |||
| pregnancy_AU = B1 | |||
| pregnancy_US = B | |||
| legal_AU = S2 | |||
| legal_US = OTC/RX | |||
| legal_status = P/POM <small>([[United Kingdom|UK]])</small> | |||
| routes_of_administration = Oral, [[intravenous therapy|IV]] | |||
=== | <!--Pharmacokinetic data--> | ||
| bioavailability = 39 to 88% | |||
| protein_bound = 15% | |||
| metabolism = [[Liver|Hepatic]] | |||
| elimination_half-life = 2–3 hours | |||
| excretion = 30–70% [[Kidney|Renal]] | |||
==== | <!--Identifiers--> | ||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 66357-35-5 | |||
| ATC_prefix = A02 | |||
| ATC_suffix = BA02 | |||
| ATC_supplemental = <br />{{ATC|A02BA|07}} (ranitidine bismuth citrate) | |||
| PubChem = 657345 | |||
| IUPHAR_ligand = 1234 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00863 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 571454 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 884KT10YB7 | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D00422 | |||
| ChEBI_Ref = {{ebicite|changed|EBI}} | |||
| ChEBI = 8776 | |||
| ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| ChEMBL = 1790041 | |||
<!--Chemical data--> | |||
| C=13 | H=22 | N=4 | O=3 | S=1 | |||
==== | | molecular_weight = 314.4 g/mol | ||
| smiles = [O-][N+](=O)\C=C(\NC)NCCSCc1oc(cc1)CN(C)C | |||
| InChI = 1/C13H22N4O3S/c1-14-13(9-17(18)19)15-6-7-21-10-12-5-4-11(20-12)8-16(2)3/h4-5,9,14-15H,6-8,10H2,1-3H3/b13-9- | |||
| InChIKey = VMXUWOKSQNHOCA-LCYFTJDEBT | |||
=== | | StdInChI_Ref = {{stdinchicite|changed|chemspider}} | ||
| StdInChI = 1S/C13H22N4O3S/c1-14-13(9-17(18)19)15-6-7-21-10-12-5-4-11(20-12)8-16(2)3/h4-5,9,14-15H,6-8,10H2,1-3H3 | |||
| StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} | |||
| StdInChIKey = VMXUWOKSQNHOCA-UHFFFAOYSA-N | |||
}} | |||
|mechAction=* Ranitidine is a competitive, reversible inhibitor of the action of histamine at the histamine H2-receptors found in gastric cells. This results in decreased gastric acid secretion and gastric volume, and reduced hydrogen ion concentration. | |||
|structure=* The active ingredient in ZANTAC 150 Tablets, ZANTAC 300 Tablets, and ZANTAC Syrup is ranitidine hydrochloride (HCl), USP, a histamine H2-receptor antagonist. It has the following structure: | |||
: [[File:Rani1.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
| | * The empirical formula is C13H22N4O3S•HCl, representing a molecular weight of 350.87. | ||
* Ranitidine HCl is a white to pale yellow, granular substance that is soluble in water. It has a slightly bitter taste and sulfur-like odor. | |||
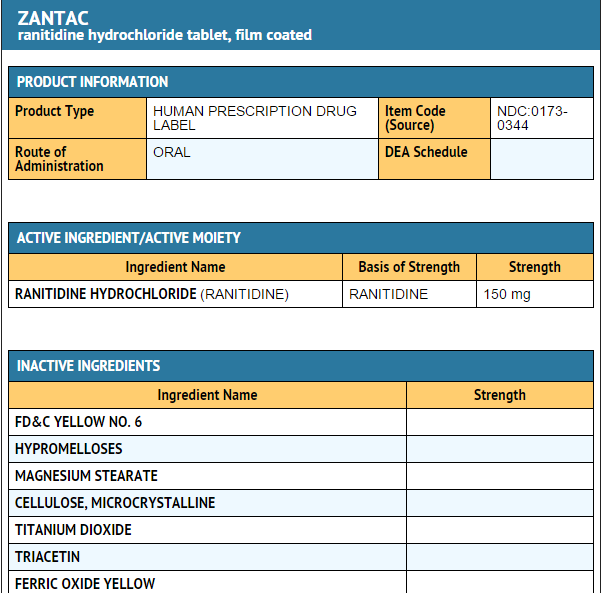
* Each ZANTAC 150 Tablet for oral administration contains 168 mg of ranitidine HCl equivalent to 150 mg of ranitidine. Each tablet also contains the inactive ingredients FD&C Yellow No. 6 Aluminum Lake, hypromellose, magnesium stearate, microcrystalline cellulose, titanium dioxide, triacetin, and yellow iron oxide. | |||
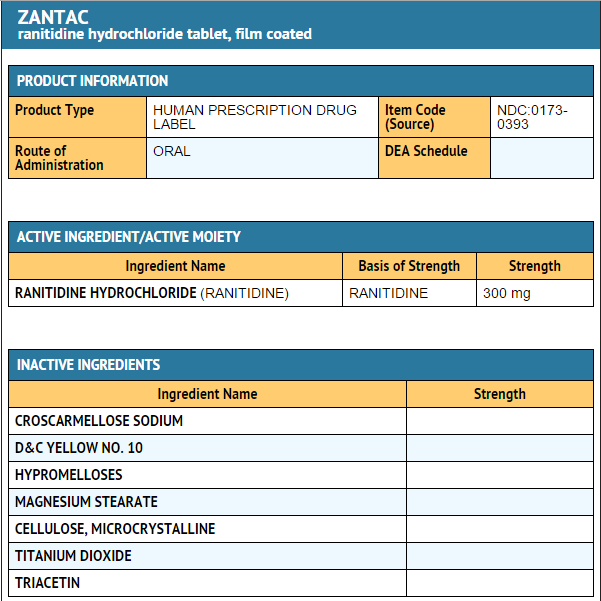
* Each ZANTAC 300 Tablet for oral administration contains 336 mg of ranitidine HCl equivalent to 300 mg of ranitidine. Each tablet also contains the inactive ingredients croscarmellose sodium, D&C Yellow No. 10 Aluminum Lake, hypromellose, magnesium stearate, microcrystalline cellulose, titanium dioxide, and triacetin. | |||
* Each 1 mL of ZANTAC Syrup contains 16.8 mg of ranitidine HCl equivalent to 15 mg of ranitidine. ZANTAC Syrup also contains the inactive ingredients alcohol (7.5%), butylparaben, dibasic sodium phosphate, hypromellose, peppermint flavor, monobasic potassium phosphate, propylparaben, purified water, saccharin sodium, sodium chloride, and sorbitol. | |||
|PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |||
|mechAction= | |||
* | |||
|structure= | |||
* | |||
: [[File: | |||
|PD= | |||
There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |||
<!--Pharmacokinetics--> | <!--Pharmacokinetics--> | ||
|PK======Absorption===== | |||
|PK= | * Oral: 50% | ||
* Protein binding: 15% | |||
=====Metabolism===== | |||
* N-oxide is the principal metabolite. | |||
* Half-life elimination: With normal renal function, ranitidine taken orally has a half-life of 2.5–3 hours. If taken intravenously, the half-life is generally 2-2.5 hours in a patient with normal creatinine clearance. | |||
=====Excretion===== | |||
|nonClinToxic= | * The primary route of excretion is the urine. In addition, approximately 30% of the orally administered dose is collected in the urine as non-absorbed drug in 24 hours. | ||
=====Elderly===== | |||
There is limited information regarding <i>Nonclinical Toxicology</i> of {{PAGENAME}} in the drug label. | * In the elderly population, the plasma half-life of ranitidine is prolonged to 3–4 hours secondary to decreased kidney function causing decreased clearance. | ||
=====Children===== | |||
* In general, studies looking pediatric patients (aged 1 month to 16 years) have showed no significant differences in pharmacokinetic parameter values in comparison to healthy adults, when correction is made for body weigh | |||
|nonClinToxic=There is limited information regarding <i>Nonclinical Toxicology</i> of {{PAGENAME}} in the drug label. | |||
<!--Clinical Studies--> | <!--Clinical Studies--> | ||
|clinicalStudies=There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | |||
|clinicalStudies= | |||
There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | |||
<!--How Supplied--> | <!--How Supplied--> | ||
|storage=* Store at 20° - 25° C (68° - 77° F) | |||
| | * Avoid excessive heat or humidity | ||
|packLabel=[[File:Zantac 2.jpg|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
* | [[File:Rani 2.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
[[File:Rani 3.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
[[File:Rani 4.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
[[File:Rani 5.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
| | [[File:Rani 6.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
[[File:Rani 7.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
|fdaPatientInfo=* Adults and children 12 years and over: | |||
:* To relieve symptoms, swallow 1 tablet with a glass of water to prevent symptoms, swallow 1 tablet with a glass of water 30 to 60 minutes before eating food or drinking beverages that cause heartburn | |||
:* Can be used up to twice daily (do not take more than 2 tablets in 24 hours) | |||
:* TAMPER EVIDENT: DO NOT USE IF THE CARTON OR PRINTED FOIL UNDER CAP IS OPEN OR TORN. | |||
|alcohol= | |alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
<!--Brand Names--> | <!--Brand Names--> | ||
|brandNames=*ZANTAC ®<ref>{{Cite web | title = ZANTAC- ranitidine hydrochloride tablet, film coated| url =http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=08010bf7-1f61-48b3-e1b5-7cecb72ba863 }}</ref> | |||
|drugShortage= | |||
}} | |||
<small>'''''Synonyms / Brand Names: Ranitidine hydrochloride, Ranitidine HCL, Ranitidine Base, Rantidine HCL, Alquen, Alter-H2, Alvidina, Apo-Ranitidin, Artomil, Azuranit, Coralen, Digestosan, Ergan, Esofex, Fendibina, Gastrial, Gastridina, Gastrolav, Gastrosedol, Kuracid, Label, Lake, Logat, Melfax, Microtid, Mideran, Neugal, Noctone, Noktome, Normon, Novo-Radinine, Nu-Ranit, Pep-Rani, Ptinolin, Quadrin, Quantor, Radin, Ran H2, Ran Lich, Rani 2, Rani AbZ, Rani-BASF, Rani-Puren, Rani-Q, Rani-Sanorania, Rani-nerton, Raniben, Raniberl, Raniberta, Ranibloc, Ranic, Ranicux, Ranidil, Ranidin, Ranidine, Ranidura, Ranifur, Ranigasan, Ranigast, Ranigen, Ranilonga, Ranimerck, Raniogas, Raniplex, Ranisan, Ranitab, Ranitic, Ranitidin, Ranitidin 1A Pharma, Ranitidin AL, Ranitidin AWD, Ranitidin Arcana, Ranitidin Atid, Ranitidin Basics, Ranitidin Duncan, Ranitidin Dyna, Ranitidin Helvepharm, Ranitidin Heumann, Ranitidin Hexal, Ranitidin Merck, Ranitidin Millet, Ranitidin NM, Ranitidin Normon, Ranitidin PB, Ranitidin Stada, Ranitidin von ct, Ranitidin-Cophar, Ranitidin-Isis, Ranitidin-ratiopharm, Ranitidina Tamarang, Ranitidina predilu Grif, Ranitiget, Ranitin, Ranitine, Ranobel, Rantacid, Ranuber, Raticina, Regalil, Renatac, Rozon, Rubiulcer, Santanol, Serviradine, Sostril, Tanidina, Taural, Terposen, Toriol, Trigger, Ulcecur, Ulcex, Ulcirex, Ulcodin, Ulcolind Rani, Ulsaven, Ultidine, Viserul, Zandid, Zantac, Zantac 150, Zantac 75, Zantac In Plastic Container, Zantarac, Zantic | |||
''''' </small> | |||
<!-- | <!--Pill Image--> | ||
<!-- | <!--Label Display Image--> | ||
<!--Category--> | <!--Category--> | ||
[[Category:Drug]] | [[Category:Drug]] | ||
[[Category:H2 receptor antagonists]] | |||
[[Category:Furans]] | |||
[[Category:Thioethers]] | |||
[[Category:Amines]] | |||
Latest revision as of 16:59, 6 May 2015
{{DrugProjectFormSinglePage |authorTag=Ammu Susheela, M.D. [1] |genericName=Ranitidine |aOrAn=a |drugClass=H2 receptor blocker |indicationType=treatment |indication=relieves heartburn associated with acid indigestion and sour stomach prevents heartburn associated with acid indigestion and sour stomach brought on by certain foods and beverages |adverseReactions=headache, constipation, diarrhea, abdominal pain |blackBoxWarningTitle=Title |blackBoxWarningBody=ConditionName:
- Content
|fdaLIADAdult=* Short-term treatment of active duodenal ulcer. Most patients heal within 4 weeks.
- Studies available to date have not assessed the safety of ranitidine in uncomplicated duodenal ulcer for periods of more than 8 weeks.
- Maintenance therapy for duodenal ulcer patients at reduced dosage after healing of acute ulcers. No placebo-controlled comparative studies have been carried out for periods of longer than 1 year.
- The treatment of pathological hypersecretory conditions (e.g., Zollinger-Ellison syndrome and systemic mastocytosis).
- Short-term treatment of active, benign gastric ulcer. Most patients heal within 6 weeks and the usefulness of further treatment has not been demonstrated. Studies available to date have not assessed the safety of ranitidine in uncomplicated, benign gastric ulcer for periods of more than 6 weeks.
- Maintenance therapy for gastric ulcer patients at reduced dosage after healing of acute ulcers. Placebo-controlled studies have been carried out for 1 year.
- Treatment of GERD. Symptomatic relief commonly occurs within 24 hours after starting therapy with ZANTAC 150 mg twice daily.
- Treatment of endoscopically diagnosed erosive esophagitis. Symptomatic relief of heartburn commonly occurs within 24 hours of therapy initiation with ZANTAC 150 mg 4 times daily.
- Maintenance of healing of erosive esophagitis. Placebo-controlled trials have been carried out for 48 weeks.
- Concomitant antacids should be given as needed for pain relief to patients with active duodenal ulcer; active, benign gastric ulcer; hypersecretory states; GERD; and erosive esophagitis.
|offLabelAdultGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Ranitidine in adult patients. |offLabelAdultNoGuideSupport=* Aspiration pneumonitis;
- Asthma.
- Duodenitis
- Gastritis medicamentosa
- Hyperchlorhydria, Nocturnal.
- Stress ulcer; Prophylaxis.
|offLabelPedGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Ranitidine (oral) in pediatric patients.
|offLabelPedNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Ranitidine (oral) in pediatric patients.
|contraindications=* Ranitidine is contraindicated for patients known to have hypersensitivity to the drug or any of the ingredients. |warnings======Allergy alert=====
- Do not use if you are allergic to ranitidine or other acid reducers
- Do not use if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition.
- With other acid reducers
- If you have kidney disease, except under the advice and supervision of a doctor
- Ask a doctor before use if you have
- Frequent chest pain
- Frequent wheezing, particularly with heartburn unexplained weight loss
- Nausea or vomiting
- Stomach pain had heartburn over 3 months. This may be a sign of a more serious condition.
- Heartburn with lightheadedness, sweating, or dizziness chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- Stop use and ask a doctor if your heartburn continues or worsens you need to take this product for more than 14 days
- If pregnant or breast-feeding, ask a health professional before use.
- Keep out of reach of children.
|FDAPregCat=B |useInPregnancyFDA=* Pregnancy Category |AUSPregCat=B1 |useInPregnancyAUS=* Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ranitidine (oral) in women who are pregnant. |useInLaborDelivery=There is no FDA guidance on use of Ranitidine (oral) during labor and delivery. |useInNursing=Infant risk cannot be ruled out. |useInPed=There is no FDA guidance on the use of Ranitidine (oral) with respect to pediatric patients. |useInGeri=There is no FDA guidance on the use of Ranitidine (oral) with respect to geriatric patients. |useInGender=There is no FDA guidance on the use of Ranitidine (oral) with respect to specific gender populations. |useInRace=There is no FDA guidance on the use of Ranitidine (oral) with respect to specific racial populations. |useInRenalImpair=There is no FDA guidance on the use of Ranitidine (oral) in patients with renal impairment. |useInHepaticImpair=There is no FDA guidance on the use of Ranitidine (oral) in patients with hepatic impairment. |useInReproPotential=There is no FDA guidance on the use of Ranitidine (oral) in women of reproductive potentials and males. |useInImmunocomp=There is no FDA guidance one the use of Ranitidine (oral) in patients who are immunocompromised.
|administration=* Oral |monitoring=There is limited information regarding Monitoring of Ranitidine (oral) in the drug label.
|IVCompat=There is limited information regarding IV Compatibility of Ranitidine (oral) in the drug label.
|drugBox={{Drugbox2 | Verifiedfields = changed | Watchedfields = changed | verifiedrevid = 458460406 | IUPAC_name = N-(2-[(5-[(dimethylamino)methyl]furan-2-yl)methylthio]ethyl)-N'-methyl-2-nitroethene-1,1-diamine; dimethyl [(5-{[(2-{[1-(methylamino)-2-nitroethenyl]amino}ethyl)sulfanyl]methyl}furan-2-yl)methyl]amine | image = Ranitidine Structural Formulae.png | width = 200 | image2 = Ranitidine-A-3D-balls.png
| tradename = Zantac | Drugs.com = Monograph | MedlinePlus = a601106 | licence_US = Ranitidine | pregnancy_AU = B1 | pregnancy_US = B | legal_AU = S2 | legal_US = OTC/RX | legal_status = P/POM (UK) | routes_of_administration = Oral, IV
| bioavailability = 39 to 88% | protein_bound = 15% | metabolism = Hepatic | elimination_half-life = 2–3 hours | excretion = 30–70% Renal
| CASNo_Ref =
| CAS_number_Ref =
| CAS_number = 66357-35-5
| ATC_prefix = A02
| ATC_suffix = BA02
| ATC_supplemental =
A02BA07 (WHO) (ranitidine bismuth citrate)
| PubChem = 657345
| IUPHAR_ligand = 1234
| DrugBank_Ref =
| DrugBank = DB00863
| ChemSpiderID_Ref =
| ChemSpiderID = 571454
| UNII_Ref =
| UNII = 884KT10YB7
| KEGG_Ref =
| KEGG = D00422
| ChEBI_Ref =
| ChEBI = 8776
| ChEMBL_Ref =
| ChEMBL = 1790041
| C=13 | H=22 | N=4 | O=3 | S=1
| molecular_weight = 314.4 g/mol
| smiles = [O-][N+](=O)\C=C(\NC)NCCSCc1oc(cc1)CN(C)C
| InChI = 1/C13H22N4O3S/c1-14-13(9-17(18)19)15-6-7-21-10-12-5-4-11(20-12)8-16(2)3/h4-5,9,14-15H,6-8,10H2,1-3H3/b13-9-
| InChIKey = VMXUWOKSQNHOCA-LCYFTJDEBT
| StdInChI_Ref =
| StdInChI = 1S/C13H22N4O3S/c1-14-13(9-17(18)19)15-6-7-21-10-12-5-4-11(20-12)8-16(2)3/h4-5,9,14-15H,6-8,10H2,1-3H3
| StdInChIKey_Ref =
| StdInChIKey = VMXUWOKSQNHOCA-UHFFFAOYSA-N
}}
|mechAction=* Ranitidine is a competitive, reversible inhibitor of the action of histamine at the histamine H2-receptors found in gastric cells. This results in decreased gastric acid secretion and gastric volume, and reduced hydrogen ion concentration.
|structure=* The active ingredient in ZANTAC 150 Tablets, ZANTAC 300 Tablets, and ZANTAC Syrup is ranitidine hydrochloride (HCl), USP, a histamine H2-receptor antagonist. It has the following structure:
- The empirical formula is C13H22N4O3S•HCl, representing a molecular weight of 350.87.
- Ranitidine HCl is a white to pale yellow, granular substance that is soluble in water. It has a slightly bitter taste and sulfur-like odor.
- Each ZANTAC 150 Tablet for oral administration contains 168 mg of ranitidine HCl equivalent to 150 mg of ranitidine. Each tablet also contains the inactive ingredients FD&C Yellow No. 6 Aluminum Lake, hypromellose, magnesium stearate, microcrystalline cellulose, titanium dioxide, triacetin, and yellow iron oxide.
- Each ZANTAC 300 Tablet for oral administration contains 336 mg of ranitidine HCl equivalent to 300 mg of ranitidine. Each tablet also contains the inactive ingredients croscarmellose sodium, D&C Yellow No. 10 Aluminum Lake, hypromellose, magnesium stearate, microcrystalline cellulose, titanium dioxide, and triacetin.
- Each 1 mL of ZANTAC Syrup contains 16.8 mg of ranitidine HCl equivalent to 15 mg of ranitidine. ZANTAC Syrup also contains the inactive ingredients alcohol (7.5%), butylparaben, dibasic sodium phosphate, hypromellose, peppermint flavor, monobasic potassium phosphate, propylparaben, purified water, saccharin sodium, sodium chloride, and sorbitol.
|PD=There is limited information regarding Pharmacodynamics of Ranitidine (oral) in the drug label.
|PK======Absorption=====
- Oral: 50%
- Protein binding: 15%
Metabolism
- N-oxide is the principal metabolite.
- Half-life elimination: With normal renal function, ranitidine taken orally has a half-life of 2.5–3 hours. If taken intravenously, the half-life is generally 2-2.5 hours in a patient with normal creatinine clearance.
Excretion
- The primary route of excretion is the urine. In addition, approximately 30% of the orally administered dose is collected in the urine as non-absorbed drug in 24 hours.
Elderly
- In the elderly population, the plasma half-life of ranitidine is prolonged to 3–4 hours secondary to decreased kidney function causing decreased clearance.
Children
- In general, studies looking pediatric patients (aged 1 month to 16 years) have showed no significant differences in pharmacokinetic parameter values in comparison to healthy adults, when correction is made for body weigh
|nonClinToxic=There is limited information regarding Nonclinical Toxicology of Ranitidine (oral) in the drug label.
|clinicalStudies=There is limited information regarding Clinical Studies of Ranitidine (oral) in the drug label.
|storage=* Store at 20° - 25° C (68° - 77° F)
- Avoid excessive heat or humidity
|packLabel=

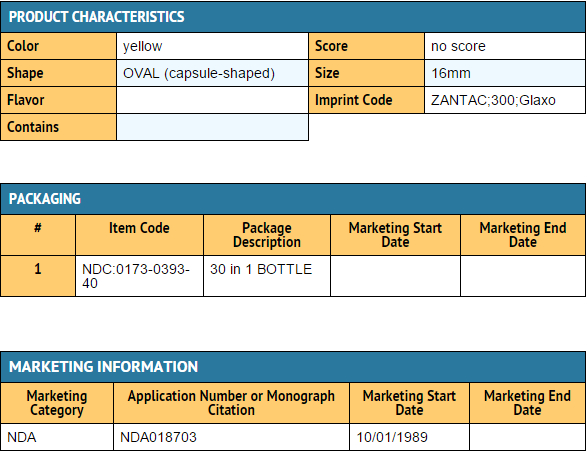
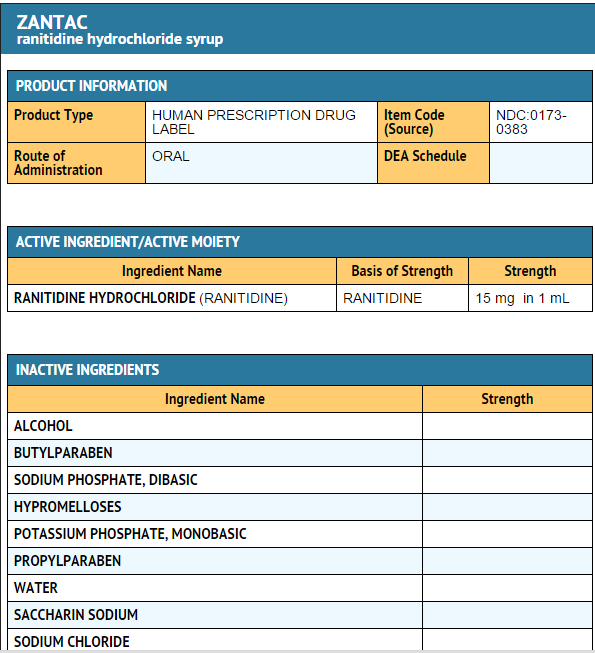
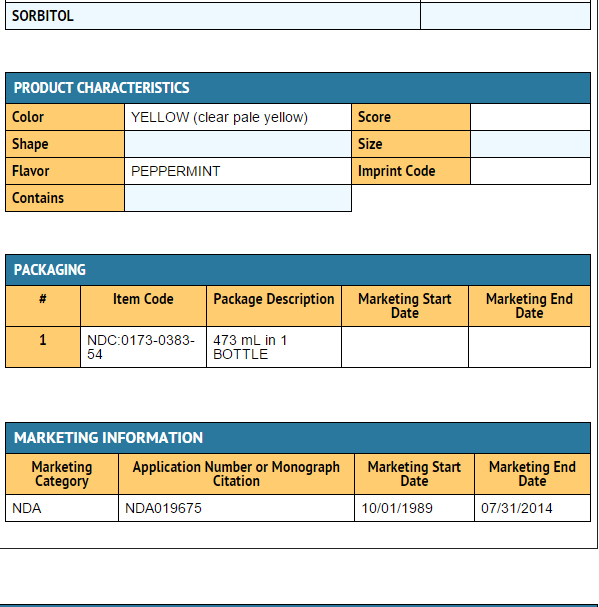
|fdaPatientInfo=* Adults and children 12 years and over:
- To relieve symptoms, swallow 1 tablet with a glass of water to prevent symptoms, swallow 1 tablet with a glass of water 30 to 60 minutes before eating food or drinking beverages that cause heartburn
- Can be used up to twice daily (do not take more than 2 tablets in 24 hours)
- TAMPER EVIDENT: DO NOT USE IF THE CARTON OR PRINTED FOIL UNDER CAP IS OPEN OR TORN.
|alcohol=* Alcohol-Ranitidine (oral) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
|brandNames=*ZANTAC ®[1] |drugShortage= }} Synonyms / Brand Names: Ranitidine hydrochloride, Ranitidine HCL, Ranitidine Base, Rantidine HCL, Alquen, Alter-H2, Alvidina, Apo-Ranitidin, Artomil, Azuranit, Coralen, Digestosan, Ergan, Esofex, Fendibina, Gastrial, Gastridina, Gastrolav, Gastrosedol, Kuracid, Label, Lake, Logat, Melfax, Microtid, Mideran, Neugal, Noctone, Noktome, Normon, Novo-Radinine, Nu-Ranit, Pep-Rani, Ptinolin, Quadrin, Quantor, Radin, Ran H2, Ran Lich, Rani 2, Rani AbZ, Rani-BASF, Rani-Puren, Rani-Q, Rani-Sanorania, Rani-nerton, Raniben, Raniberl, Raniberta, Ranibloc, Ranic, Ranicux, Ranidil, Ranidin, Ranidine, Ranidura, Ranifur, Ranigasan, Ranigast, Ranigen, Ranilonga, Ranimerck, Raniogas, Raniplex, Ranisan, Ranitab, Ranitic, Ranitidin, Ranitidin 1A Pharma, Ranitidin AL, Ranitidin AWD, Ranitidin Arcana, Ranitidin Atid, Ranitidin Basics, Ranitidin Duncan, Ranitidin Dyna, Ranitidin Helvepharm, Ranitidin Heumann, Ranitidin Hexal, Ranitidin Merck, Ranitidin Millet, Ranitidin NM, Ranitidin Normon, Ranitidin PB, Ranitidin Stada, Ranitidin von ct, Ranitidin-Cophar, Ranitidin-Isis, Ranitidin-ratiopharm, Ranitidina Tamarang, Ranitidina predilu Grif, Ranitiget, Ranitin, Ranitine, Ranobel, Rantacid, Ranuber, Raticina, Regalil, Renatac, Rozon, Rubiulcer, Santanol, Serviradine, Sostril, Tanidina, Taural, Terposen, Toriol, Trigger, Ulcecur, Ulcex, Ulcirex, Ulcodin, Ulcolind Rani, Ulsaven, Ultidine, Viserul, Zandid, Zantac, Zantac 150, Zantac 75, Zantac In Plastic Container, Zantarac, Zantic