Prazosin labels and packages: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
No edit summary |
||
| Line 2: | Line 2: | ||
{{Prazosin}} | {{Prazosin}} | ||
{{CMG}}; {{AE}} {{AK}} | {{CMG}}; {{AE}} {{AK}} | ||
==Prazosin Labels | ==Prazosin Labels and Packages== | ||
PRINCIPAL DISPLAY PANEL - 1 mg Capsule Bottle Label | PRINCIPAL DISPLAY PANEL - 1 mg Capsule Bottle Label | ||
Revision as of 19:57, 12 March 2014
| Prazosin |
|---|
| Prazosin®, Minipress® FDA Package Insert |
| Indications and Usage |
| Dosage and Administration |
| Contraindications |
| Warnings |
| Precautions |
| Adverse Reactions |
| Drug Interactions |
| Use in Specific Populations |
| Overdosage |
| Description |
| Clinical Pharmacology |
| Nonclinical Toxicology |
| How Supplied/Storage and Handling |
| Patient Counseling Information |
| Labels and Packages |
| Clinical Trials on Prazosin |
| ClinicalTrials.gov |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Abdurahman Khalil, M.D. [2]
Prazosin Labels and Packages
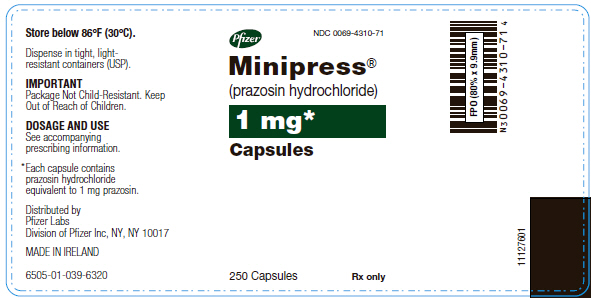
PRINCIPAL DISPLAY PANEL - 1 mg Capsule Bottle Label
Pfizer NDC 0069-4310-71
Minipress® (prazosin hydrochloride)
1 mg* Capsules
250 Capsules Rx only
 |
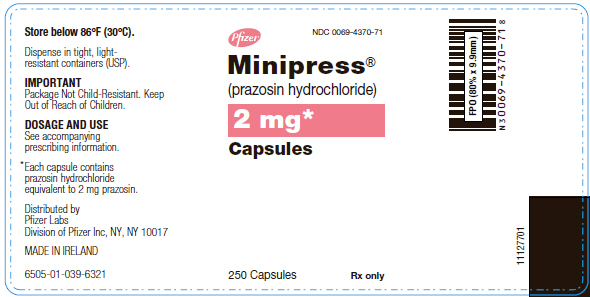
PRINCIPAL DISPLAY PANEL - 2 mg Capsule Bottle Label
Pfizer NDC 0069-4370-71
Minipress® (prazosin hydrochloride)
2 mg* Capsules
250 Capsules Rx only
 |
PRINCIPAL DISPLAY PANEL - 5 mg Capsule Bottle Label
Pfizer NDC 0069-4380-71
Minipress® (prazosin hydrochloride)
5 mg* Capsules
250 Capsules Rx only

References
- ↑ "MINIPRESS (PRAZOSIN HYDROCHLORIDE) CAPSULE [PFIZER LABORATORIES DIV PFIZER INC]". Retrieved 6 March 2014.