Polyethylene: Difference between revisions
No edit summary |
No edit summary |
||
| Line 3: | Line 3: | ||
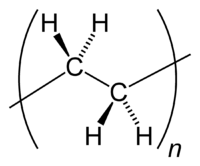
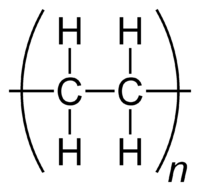
[[Image:Polyethylene-repeat-2D-flat.png|thumb|right|200px|A simpler way of representing the [[repeating unit]]. Note, however, that the C−H [[Molecular geometry|bond angles]] are not 90° as this diagram would indicate, but are approximately 110°, since each [[carbon]] atom is [[tetrahedron|tetrahedral]] ([[Orbital hybridisation|sp<sup>3</sup>]]).]] | [[Image:Polyethylene-repeat-2D-flat.png|thumb|right|200px|A simpler way of representing the [[repeating unit]]. Note, however, that the C−H [[Molecular geometry|bond angles]] are not 90° as this diagram would indicate, but are approximately 110°, since each [[carbon]] atom is [[tetrahedron|tetrahedral]] ([[Orbital hybridisation|sp<sup>3</sup>]]).]] | ||
{{SI}} | {{SI}} | ||
{{EH}} | {{EH}} | ||
| Line 117: | Line 116: | ||
{{WS}} | {{WS}} | ||
{{WH}} | {{WH}} | ||
{{jb1}} | |||
Latest revision as of 19:29, 18 June 2009
|
WikiDoc Resources for Polyethylene |
|
Articles |
|---|
|
Most recent articles on Polyethylene Most cited articles on Polyethylene |
|
Media |
|
Powerpoint slides on Polyethylene |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Polyethylene at Clinical Trials.gov Clinical Trials on Polyethylene at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Polyethylene
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Polyethylene Discussion groups on Polyethylene Patient Handouts on Polyethylene Directions to Hospitals Treating Polyethylene Risk calculators and risk factors for Polyethylene
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Polyethylene |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [1] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Polyethylene (IUPAC name polyethene) is a thermoplastic commodity heavily used in consumer products. Over 60 million tons of the material are produced worldwide every year.
Description
Polyethylene is a polymer consisting of long chains of the monomer ethylene (IUPAC name ethene). The recommended scientific name 'polyethene' is systematically derived from the scientific name of the monomer.[1][2] In certain circumstances it is useful to use a structure–based nomenclature. In such cases IUPAC recommends poly(methylene).[2] The difference is due to the 'opening up' of the monomer's double bond upon polymerisation.
In the polymer industry the name is sometimes shortened to PE, in a manner similar to that by which other polymers like polypropylene and polystyrene are shortened to PP and PS, respectively. In the United Kingdom the polymer is commonly called polythene, although this is not recognised scientifically.
The ethene molecule (known almost universally by its common name ethylene), C2H4 is CH2=CH2, Two CH2 groups connected by a double bond, thus:
 File:Ethylene-3D-vdW.png
File:Ethylene-3D-vdW.pngPolyethylene is created through polymerization of ethene. It can be produced through radical polymerization, anionic addition polymerization, ion coordination polymerization or cationic addition polymerization. This is because ethene does not have any substituent groups that influence the stability of the propagation head of the polymer. Each of these methods results in a different type of polyethylene.
Classification of polyethylenes
Polyethylene is classified into several different categories based mostly on its density and branching. The mechanical properties of PE depend significantly on variables such as the extent and type of branching, the crystal structure, and the molecular weight.
- Ultra high molecular weight polyethylene (UHMWPE)
- Ultra low molecular weight polyethylene (ULMWPE - PE-WAX)
- High molecular weight polyethylene (HMWPE)
- High density polyethylene (HDPE)
- High density cross-linked polyethylene (HDXLPE)
- Cross-linked polyethylene (PEX)
- Medium density polyethylene (MDPE)
- Low density polyethylene (LDPE)
- Linear low density polyethylene (LLDPE)
- Very low density polyethylene (VLDPE)
UHMWPE is polyethylene with a molecular weight numbering in the millions, usually between 3.1 and 5.67 million. The high molecular weight results in less efficient packing of the chains into the crystal structure as evidenced by densities less than high density polyethylene (e.g. 0.930 - 0.935 g/cm3). The high molecular weight results in a very tough material. UHMWPE can be made through any catalyst technology, although Ziegler catalysts are most common. Because of its outstanding toughness, cut, wear and excellent chemical resistance, UHWMPE is used in a wide diversity of applications. These include can and bottle handling machine parts, moving parts on weaving machines, bearings, gears, artificial joints, edge protection on ice rinks, butchers' chopping boards. It competes with Aramid in bulletproof vests as Spectra (or Dyneema) fibers.
HDPE is defined by a density of greater or equal to 0.941 g/cm3. HDPE has a low degree of branching and thus stronger intermolecular forces and tensile strength. HDPE can be produced by chromium/silica catalysts, Ziegler-Natta catalysts or metallocene catalysts. The lack of branching is ensured by an appropriate choice of catalyst (e.g. chromium catalysts or Ziegler-Natta catalysts) and reaction conditions. HDPE is used in products and packaging such as milk jugs, detergent bottles, margarine tubs, garbage containers and water pipes.
HDPE is also widely used in the fireworks community. In tubes of varying length (depending on the size of the ordnance), HDPE is used as a replacement for the supplied cardboard mortar tubes for two primary reasons. One, it is much safer than the supplied cardboard tubes because if a shell were to malfunction and explode inside (flower pot) an HDPE tube, the tube will not shatter. The second reason is that they are reusable allowing designers to create multiple shot mortar racks. Pyrotechnicians discourage the use of PVC tubing in mortar tubes because it tends to shatter, sending shards of plastic at possible spectators, and will not show up in x-rays.
PEX is a medium- to high-density polyethylene containing cross-link bonds introduced into the polymer structure, changing the thermoplast into an elastomer. The high-temperature properties of the polymer are improved, its flow is reduced and its chemical resistance is enhanced. PEX is used in some potable water plumbing systems, as tubes made of the material can be expanded to fit over a metal nipple, and it will slowly return to its original shape, forming a permanent, water-tight connection.
MDPE is defined by a density range of 0.926 - 0.940 g/cm3. MDPE can be produced by chromium/silica catalysts, Ziegler-Natta catalysts or metallocene catalysts.MDPE has good shock and drop resistance properties. It also is less notch sensitive than HDPE, stress cracking resistance is better than HDPE. MDPE is typically used in gas pipes and fittings, sacks, shrink film, packaging film, carrier bags, screw closures.
LLDPE is defined by a density range of 0.915 - 0.925 g/cm3. is a substantially linear polymer, with significant numbers of short branches, commonly made by copolymerization of ethylene with short-chain alpha-olefins (e.g. 1-butene, 1-hexene, and 1-octene). LLDPE has higher tensile strength than LDPE. Exhibits higher impact and puncture resistance than LDPE. Lower thickness (gauge) films can be blown compared to LDPE, with better environmental stress cracking resistance compared to LDPE but is not as easy to process. LLDPE is used in packaging, particularly film for bags and sheets. Lower thickness (gauge) may be used compared to LDPE. Cable covering, toys, lids, buckets and containers, pipe. While other applications are available, LLDPE is used predominantly in film applications due to its toughness, flexibility, and relative transparency.
LDPE is defined by a density range of 0.910 - 0.940 g/cm3. LDPE has a high degree of short and long chain branching, which means that the chains do not pack into the crystal structure as well. It has therefore less strong intermolecular forces as the instantaneous-dipole induced-dipole attraction is less. This results in a lower tensile strength and increased ductility. LDPE is created by free radical polymerization. The high degree of branches with long chains gives molten LDPE unique and desirable flow properties. LDPE is used for both rigid containers and plastic film applications such as plastic bags and film wrap.
VLDPE is defined by a density range of 0.880 - 0.915 g/cm3. is a substantially linear polymer, with high levels of short chain branches, commonly made by copolymerization of ethylene with short-chain alpha-olefins (e.g. 1-butene, 1-hexene, and 1-octene). VLDPE is most commonly produced using metallocene catalysts due to the greater co-monomer incorporation exhibited by these catalysts. VLDPE’s are used for hose and tubing, ice and frozen food bags, food packaging and stretch wrap, as well as impact modifiers when blended with other polymers.
Recently, much research activity has focused on the nature and distribution of long chain branches in polyethylene. In HDPE, a relatively small number of these branches, perhaps 1 in 100 or 1,000 branches per backbone carbon, can significantly affect the rheological properties of the polymer.
Ethylene copolymers
In addition to copolymerization with alpha-olefins, ethylene can also be copolymerized with a wide range of other monomers and ionic composition that creates ionized free radicals. Common examples include vinyl acetate (resulting product is ethylene-vinyl acetate copolymer, or EVA, widely used in athletic shoe sole foams), and a variety of acrylates (applications include packaging and sporting goods).
History
Polyethylene was first synthesized by the German chemist Hans von Pechmann, who prepared it by accident in 1898 while heating diazomethane. When his colleagues Eugen Bamberger and Friedrich Tschirner characterized the white, waxy substance he had created, they recognized that it contained long -CH2- chains and termed it polymethylene.
The first industrially practical polyethylene synthesis was discovered (again by accident) in 1933 by Eric Fawcett and Reginald Gibson at the ICI works in Northwich, England.[3] Upon applying extremely high pressure (several hundred atmospheres) to a mixture of ethylene and benzaldehyde, they again produced a white waxy material. Because the reaction had been initiated by trace oxygen contamination in their apparatus, the experiment was at first difficult to reproduce. It was not until 1935 that another ICI chemist, Michael Perrin, developed this accident into a reproducible high-pressure synthesis for polyethylene that became the basis for industrial LDPE production beginning in 1939.
Subsequent landmarks in polyethylene synthesis have revolved around the development of several types of catalyst that promote ethylene polymerization at more mild temperatures and pressures. The first of these was a chromium trioxide based catalyst discovered in 1951 by Robert Banks and J. Paul Hogan at Phillips Petroleum. In 1953, the German chemist Karl Ziegler developed a catalytic system based on titanium halides and organoaluminum compounds that worked at even milder conditions than the Phillips catalyst. The Phillips catalyst is less expensive and easier to work with, however, and both methods are used in industrial practice.
By the end of the 1950s both the Phillips and Ziegler type catalysts were being used for HDPE production. Phillips' initially had difficulties producing a HDPE product of uniform quality, and filled warehouses with off-specification plastic. However, financial ruin was unexpectedly averted in 1957, when the hula hoop, a toy consisting of a circular polyethylene tube, became a fad among youth in the United States.
A third type of catalytic system, one based on metallocenes, was discovered in 1976 in Germany by Walter Kaminsky and Hansjörg Sinn. The Ziegler and metallocene catalyst families have since proven to be very flexible at copolymerizing ethylene with other olefins and have become the basis for the wide range of polyethylene resins available today, including very low density polyethylene , and linear low density polyethylene . Such resins, in the form of fibers like Dyneema, have (as of 2005) begun to replace aramids in many high-strength applications.
Until recently, the metallocenes were the most active single-site catalysts for ethylene polymerisation known - new catalysts are typically compared to zirconocene dichloride. Much effort is currently being exerted on developing new single-site (so-called post-metallocene) catalysts, that may allow greater tuning of the polymer structure than is possible with metallocenes. Recently, work by Fujita at the Mitsui corporation (amongst others) has demonstrated that certain salicylaldimine complexes of Group 4 metals show substantially higher activity than the metallocenes.
Physical properties
Depending on the crystallinity and molecular weight, a melting point and glass transition may or may not be observable. The temperature at which these occur varies strongly with the type of polyethylene. For common commercial grades of medium-density and high-density polyethylene, the melting point is typically in the range 120-130 °C. The melt point for average commercial low-density polyethylene is typically 105-115 °C. Most LDPE, MDPE, and HDPE grades have excellent chemical resistance and do not dissolve at room temperature because of the crystallinity. Polyethylene (other than cross-linked polyethylene) usually can be dissolved at elevated temperatures in aromatic hydrocarbons, such as toluene or xylene, or chlorinated solvents, such as trichloroethane or trichlorobenzene.
References
External links
Template:SIB Template:Plastics
bg:Полиетилен ca:Polietilè cs:Polyethylen da:Polyethylen de:Polyethylen it:Polietilene la:Polyethylenum lt:Polietilenas ms:Polietilena nl:Polyetheen sq:Polietileni simple:Polyethylene sk:Polyetylén fi:Polyeteeni sv:Polyeten uk:Поліетилен
- ↑ A Guide to IUPAC Nomenclature of Organic Compounds, Blackwell Scientific Publications, Oxford (1993).
- ↑ 2.0 2.1 J. KAHOVEC, R. B. FOX and K. HATADA; “Nomenclature of regular single-strand organic polymers (IUPAC Recommendations 2002);” Pure and Applied Chemistry; IUPAC; 2002; 74 (10): pp. 1921–1956.
- ↑ "Winnington history in the making". This is Cheshire. Retrieved 2006-12-05.