Polycystic ovary syndrome: Difference between revisions
No edit summary |
No edit summary |
||
| Line 15: | Line 15: | ||
{{CMG}} | {{CMG}} | ||
{{SK}} Stein-Leventhal syndrome; PCOS; polycystic ovary disease; PCOD | {{SK}} Stein-Leventhal syndrome; PCOS; polycystic ovary disease; PCOD; syndrome O; syndrome X; functional ovarian hyperandrogenism; hyperandrogenic chronic anovulation; ovarian dysmetabolic syndrome | ||
==Overview== | ==Overview== | ||
Revision as of 16:08, 24 August 2012
Template:DiseaseDisorder infobox Template:Search infobox
For patient information click here.
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Synonyms and keywords: Stein-Leventhal syndrome; PCOS; polycystic ovary disease; PCOD; syndrome O; syndrome X; functional ovarian hyperandrogenism; hyperandrogenic chronic anovulation; ovarian dysmetabolic syndrome
Overview
Polycystic Ovary Syndrome is an endocrine disorder that affects approximately one in ten women.[1] It occurs amongst all races and nationalities, is the most common hormonal disorder among women of reproductive age, and is a leading cause of infertility. The principal features are weight issues, lack of regular ovulation and/or menstrual and excessive amounts or effects of androgenic (masculinizing) hormones. The symptoms and severity of the syndrome vary greatly between women. While the causes are unknown, insulin resistance, diabetes and obesity are all strongly correlated with PCOS.
Nomenclature
Other names for this syndrome include
- Polycystic Ovary Disease (PCOD)
- Premature Ovarian Failure (POF)
- Syndrome O
- Syndrome X
- Functional Ovarian Hyperandrogenism
- Hyperandrogenic Chronic Anovulation
- Ovarian Dysmetabolic Syndrome
Definition
Two definitions are commonly used:
- In 1990 a consensus workshop sponsored by the NIH/NICHD suggested that a patient has PCOS if she has (1) signs of androgen excess (clinical or biochemical), (2) oligoovulation, and (3) other entities are excluded that would cause polycystic ovaries.
- In 2003 a consensus workshop sponsored by ESHRE/ASRM in Rotterdam indicated PCOS to be present if 2 out of 3 criteria are met: (1) oligoovulation and/or anovulation, (2) excess androgen activity, (3) polycystic ovaries (by gynecologic ultrasound), and other causes of PCOS are excluded.
The Rotterdam definition is wider, including many more patients, notably patients without androgen excess, whereas in the NIH/NICHD definition androgen excess is a prerequisite. Critics maintain that findings obtained from the study of patients with androgen excess cannot necessarily be extrapolated to patients without androgen excess.
Signs and symptoms
Common symptoms of PCOS include
- Ovarian Cysts — (also called Polcystic Ovaries or PCO) is not a necessary symptom to be 'PCOS', many do not have cyst issues. Likewise, having cysts does not prove PCOS as many women without any medical problems have cysts at some point in their lives.
- Oligomenorrhea, amenorrhea — irregular, few, or absent menstrual periods; cycles that do occur may be heavy (heavy bleeding is also an early warning sign of endometrial cancer(in post menopausal women), for which women with PCOS are at slightly higher risk)
- Infertility, generally resulting from chronic anovulation (lack of ovulation)
- Elevated serum (blood) levels of androgens (male hormones), specifically testosterone, androstenedione, and dehydroepiandrosterone sulfate (DHEAS), causing hirsutism and occasionally masculinization
- Dyspareunia — pain during sexual intercourse
- Androgenic alopecia — male-pattern baldness
- Acne, oily skin, seborrhea
- Acanthosis nigricans — dark patches of skin, tan to dark brown or black, a sign of insulin resistance, which is associated with PCOS
- Acrochordons (skin tags) — tiny flaps of skin
- Prolonged periods of PMS-like symptoms (bloating, mood swings, pelvic pain, backaches)
- Sleep apnea
Mild symptoms of hyperandrogenism, such as acne or hyperseborrhea, are frequent in adolescent girls and are often associated with irregular menstrual cycles. In most instances, these symptoms are transient and only reflect the immaturity of the hypothalamic-pituitary-ovary axis during the first years following menarche.[2]
Signs are:
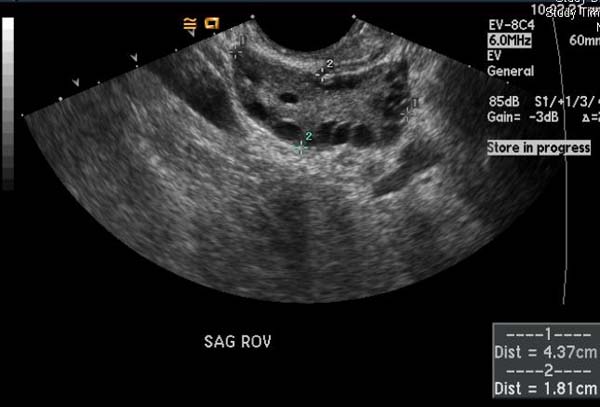
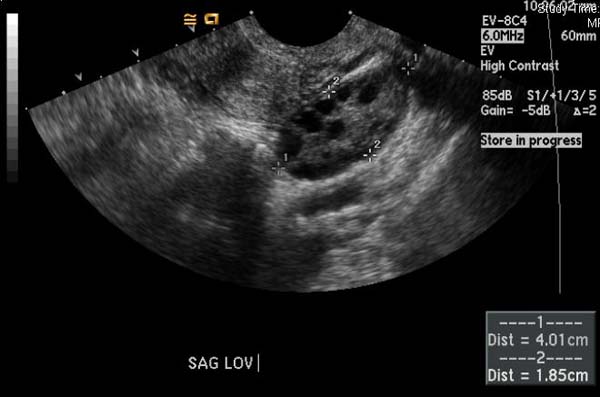
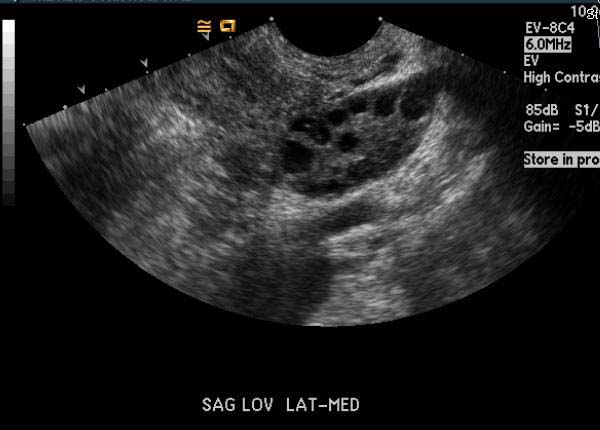
- Multiple small follicles on the ovaries (10 or more) (one form of ovarian cyst). Sonographically they may look like a string of pearls.
- Enlarged ovaries, generally 1.5 to 3 times larger than normal(volume >20mls), resulting from multiple peripherally located small follicles and echogenic ovarian stroma.
- Thickened, smooth, pearl-white outer surface of ovary on laparoscopic examination.
- The ratio of LH (Luteinizing hormone) to FSH (Follicle stimulating hormone) is greater than 1:1, as tested on Day 3 of the menstrual cycle.
- High levels of testosterone.
- Low levels of sex hormone binding globulin.
- Hyperinsulinemia.
It is important to know that PCOS can present in any age. Many can be diagnosed as young children, some might not present until after menopause. It is vital to find a PCOS knowledgeable doctor to catch this disorder as many miss the diagnoses - sometimes for years.
Risks
Women with PCOS are at risk for the following:
- Endometrial hyperplasia and endometrial cancer (cancer of the uterine lining) are possible, due to overaccumulation of uterine lining, and also lack of progesterone resulting in prolonged stimulation of uterine cells by estrogen
- Insulin resistance/Type II diabetes
- High blood pressure
- Dyslipidemia (disorders of lipid metabolism — cholesterol and triglycerides)
- Cardiovascular disease
- Strokes
- Weight gain
- Miscarriage
Diagnosis
It is vital to note that not all women with PCOS have polycystic ovaries (PCO), nor do all women with ovarian cysts have PCOS; although a pelvic ultrasound is a major diagnostic tool, it is not the only one. Diagnosis can be difficult, particularly because of the wide range of symptoms and the variability in presentation (which is why this disorder is characterized as a syndrome rather than a disease).
- Standard diagnostic assessments:
- History-taking, specifically for menstrual pattern, obesity, hirsutism, and the absence of breast discharge. A clinical prediction rule found that these four questions (http://www.cfp.ca/cgi/content/full/53/6/1041/T50531041) can diagnose PCOS with a sensitivity of 85% and a specificity of 85%.[3]
- Gynecologic ultrasonography, specifically looking for ovarian cysts
- Blood testosterone level: free testosterone is more sensitive than total; free androgen index is often used as a substitute.
- Common assessments for associated conditions or risks
- Fasting biochemical screen and lipid profile
- 2-hour oral glucose tolerance test (GTT) in patients with risk factors (obesity, family history, history of gestational diabetes) and may indicate impaired glucose tolerance (insulin resistance) in 15-30% of women with PCOS. Frank diabetes can be seen in 65–68% of women with this condition. Insulin resistance can be observed in both normal weight and overweight patients.
- For exclusion of other disorders that may cause similar symptoms:
- Prolactin to rule out hyperprolactinemia
- TSH to rule out hypothyroidism
- 17-hydroxyprogesterone to rule out 21-hydroxylase deficiency (CAH). Many such women may appear similar to PCOS and be made worse by insulin resistance or obesity, but they can be greatly helped by adrenal suppression with low-dose glucocorticoid therapy.
- DHEAS
- Androstenedione
The role of other tests is more controversial, including:
- Fasting insulin level or GTT with insulin levels (also called IGTT). Elevated insulin levels have been helpful to predict response to medication and may indicate women who will need higher dosages of Metformin or the use of a second medication to significantly lower insulin levels. Elevated blood sugar and insulin values do not predict who responds to an insulin-lowering medication, low-glycemic diet, and exercise. Many women with normal levels may benefit from combination therapy. A hypoglycemic response in which the two-hour insulin level is higher and the blood sugar lower than fasting is consistent with insulin resistance. A mathematical derivation known as the HOMAI, calculated from the fasting values in glucose and insulin concentrations, allows a direct and moderately accurate measure of insulin sensitivity.
- LH:FSH ratio elevation; FSH normal or low, LH high. Measurement of LH and FSH is easy and fairly standard, but the pattern is not very specific and is seen in only a subset of patients; present in less than 50% in one study.[4]
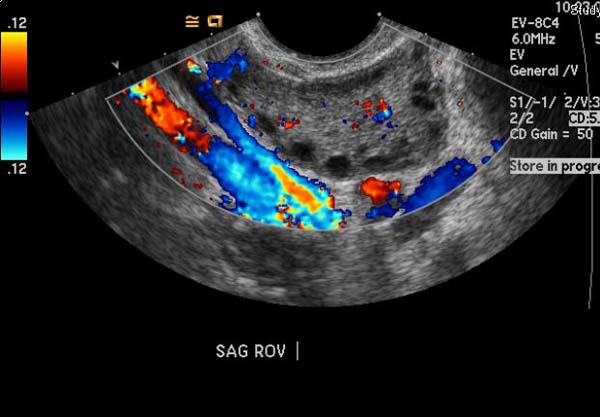
Ultrasonography
(Images courtesy of RadsWiki)
-
Polycystic ovary syndrome
-
Polycystic ovary syndrome
-
Polycystic ovary syndrome
-
Polycystic ovary syndrome
Differential diagnosis
Other causes of irregular or absent menstruation and hirsutism, such as congenital adrenal hyperplasia, Cushing's syndrome, hyperprolactinemia, and other pituitary or adrenal disorders, should be investigated. PCOS has been reported in other insulin resistant situations such as acromegaly.
Pathogenesis
Polycystic Ovaries develop when the ovaries are stimulated to produce excessive amounts of male hormones (androgens), particularly testosterone, either through the release of excessive luteinizing hormone (LH) by the anterior pituitary gland or through high levels of insulin in the blood (hyperinsulinaemia) in women whose ovaries are sensitive to this stimulus.
This syndrome acquired its most widely used name because a common sign is multiple (poly) ovarian cysts. These form where egg follicles matured but were never released from the ovary because of abnormal hormone levels. These generally take on a 'string of pearls' appearance. The condition was first described in 1935 by Dr. Stein and Dr. Leventhal, hence its original name of Stein-Leventhal syndrome.
PCOS is characterized by a complex set of symptoms, and the cause cannot be determined for all patients. However, research to date suggests that insulin resistance could be a leading cause. PCOS may also have a genetic predisposition, and further research into this possibility is taking place. No specific gene has been identified, and it is thought that many genes could contribute to the development of PCOS.
A majority of patients with PCOS have insulin resistance. Their elevated insulin levels contribute to or cause the abnormalities seen in the hypothalamic-pituitary-ovarian axis that lead to PCOS.
Specifically, hyperinsulinemia increases GnRH pulse frequency, LH over FSH dominance, increased ovarian androgen production, decreased follicular maturation, and decreased SHBG binding; all these steps lead to the development of PCOS. Insulin resistance is a common finding among both normal weight and overweight PCOS patients.
PCOS may be associated with chronic inflammation, with several investigators correlating inflammatory mediators with anovulation and other PCOS symptoms.[5][6]
Treatment
Medical treatment of PCOS is tailored to the patient's goals. Broadly, these may be considered under three categories:
- Restoration of fertility
- Treatment of hirsutism or acne
- Restoration of regular menstruation, and prevention of endometrial hyperplasia and endometrial cancer
In each of these areas, there is considerable debate as to the optimal treatment. One of the major reasons for this is the lack of large scale clinical trials comparing different treatments. Smaller trials tend to be less reliable, and hence may produce conflicting results.
General interventions that help to reduce weight or insulin resistance can be beneficial for all these aims, because they address what is believed to be the underlying cause of the syndrome. Where PCOS is associated with overweight or obesity, successful weight loss is probably the most effective method of restoring normal ovulation/menstruation, but many women find it very difficult to achieve and sustain significant weight loss. Low-carbohydrate diets and sustained regular exercise may help, and some experts recommend a low-GI diet in which a significant part of the total carbohydrates are obtained from fruit, vegetables and wholegrain sources.
Many women find insulin-lowering medications such as metformin hydrochloride (Glucophage®), pioglitazone hydrochloride (Actos®), and rosiglitazone maleate (Avandia®) helpful, and ovulation may resume when they use these agents. Many women report that metformin use is associated with upset stomach, diarrhea, and weight-loss. Such side effects usually resolve within 2–3 weeks. Starting with a lower dosage and gradually increasing the dosage over 2–3 weeks and taking the medication toward the end of a meal may reduce side effects. It may take up to six months to see results, but when combined with exercise and a low glycemic index diet up to 85% will improve menstrual cycle regularity and ovulation.
Treatment of Infertility
Clomiphene citrate and metformin are the principal treatments used to help infertility. Both have been shown to be effective, but in the largest trial to date clomiphene appeared to be most effective. [7] In this trial, 626 women were randomized to three groups: metformin alone, clomiphene alone, or both. The live birth rates after 6 months were 7.2% (metformin), 22.5% (clomiphene), and 26.8% (both). The major complication of clomiphene was multiple pregancy, affecting 0%, 6% and 3.1% of women respectively. The overall success rates for live birth remained disappointing, even in women receiving combined therapy, but it is important to consider that the women in this trial had already been attempting to conceive for an average of 3.5 years, and over half had received previous treatment for infertility. Thus, these were women with significant fertility problems, and the live birth rates are probably not representative of the 'average' PCOS woman.
However, many specialists continue to recommend metformin which has, separately, been shown to increase ovulation rates [8] and reduce miscarriage rates.[9]. Metformin may be a rational choice in women in whom significant insulin resistance is diagnosed or suspected, as clomiphene works through a different mechanism and does not affect insulin resistance.
Diet adjustments and weight loss also increase rates of pregnancy. The most drastic increase in ovulation rate occurs with a combination of diet modification, weight loss, and treatment with metformin and clomiphene citrate[10]. It is currently unknown if diet change and weight loss alone have an effect on live birth rates comparable to those reported with clomiphene and metformin.
Though the use of basal body temperature or BBT charts is sometimes advised to predict ovulation, clinical trials have not supported a useful role.
For patients who do not respond to clomiphene, metformin, other insulin-sensitizing agents, diet and lifestyle modification, there are options available including assisted reproductive technology procedures such as controlled ovarian hyperstimulation and in vitro fertilisation. Ovarian stimulation has an associated risk of ovarian hyperstimulation in women with PCOS — a dangerous condition with morbidity and rare mortality. Thus recent developments have allowed the oocytes present in the multiple follicles to extracted in natural, unstimulated cycles and then matured in vitro, prior to IVF. This technique is known as IVM (in-vitro-maturation)
Though surgery is usually the treatment option of last resort, the polycystic ovaries can be treated with surgical procedures such as
- laparoscopy electrocauterization or laser cauterization
- ovarian wedge resection (rarely done now because it is more invasive and has a 30% risk of adhesions, sometimes very severe, which can impair fertility) was an older therapy
- ovarian drilling
Treatment of Hirsutism and Acne
Cyproterone acetate is an anti-androgen, which blocks the action of male hormones that are believed to contribute to acne and the growth of unwanted facial and body hair. Cyproterone acetate is also contained in the contraceptive pill Dianette®. Spironolactone also has some benefits, again through anti-androgen activity, and metformin can also help. Eflornithine is a drug which is applied to the skin in cream form (Vaniqa®), and acts directly on the hair follicles to inhibit hair growth. It is usually applied to the face.
Although all of these agents have shown some efficacy in clinical trials, the average reduction in hair growth is generally in the region of 25%, which may not be enough to eliminate the social embarrassment of hirsutism, or the inconvenience of plucking/shaving. Individuals may vary in their response to different therapies, and it is usually worth trying other drug treatments if one does not work, but drug treatments do not work well for all individuals. Alternatives include electrolysis and various forms of laser therapy.
Treatment of Menstrual Irregularity and Prevention of Endometrial Hyperplasia/Cancer
If fertility is not the primary aim, then menstruation can usually be regularised with a contraceptive pill. Most brands of contraceptive pill result in a withdrawal bleed every 28 days. Dianette® (a contraceptive pill containing cyproterone acetate) is also beneficial for hirsutism, and is therefore often prescribed in PCOS.
If a regular menstrual cycle is not desired, then a standard contraceptive pill is not appropriate. Women who are having irregular menses do not necessarily require any therapy; most experts consider that if a menstrual bleed occurs at least every three months, then the endometrium (womb lining) is being shed sufficiently often to prevent an increased risk of endometrial abnormalities or cancer. If menstruation occurs less often or not at all, some form of progestogen replacement is recommended. Some women prefer a uterine progestogen implant such as the Mirena® coil, which provides simultaneous contraception and endometrial protection for years, though often with unpredictable minor bleeding. An alternative is oral progestogen taken at intervals (e.g. every three months) to induce a predictable menstrual bleed.
Alternative approaches
D-chiro-inositol (DCI) offers a well-tolerated and effective alternative treatment for PCOS. It has been evaluated in two peer-reviewed, double-blind studies and found to help both lean and obese women with PCOS; diminishing many of the primary clinical presentations of PCOS.[11] [12] It has no known side-effects and is a naturally occurring human metabolite known to be involved in insulin metabolism.[13] Contrary to common — but false — claims, DCI is not a drug but rather a nutrient (as defined by the DSHEA) and is commercially available as a nutritional supplement in the USA.
Ian Stoakes, a UK-based scientist has recently claimed some success in treating PCOS through tailored diets, believing that there is a strong link between PCOS, diabetes (and associated diseases) and inflammation caused by the failure of the blood to absorb specific foods. Blood samples are tested to see how they react to different food types to provide the patient with a list of foods she can eat and foods to avoid. Weight loss, alleviation of symptoms and successful pregnancies are claimed for this approach. It remains a totally unproven approach with no research papers listed in PubMed by Stoakes concerning PCOS.
References
- Ehrmann DA. Polycystic ovary syndrome. N Engl J Med 2005;352:1223-36. PMID 15788499.
- (UK) Royal College of Obstetricians and Gynaecologists. Guideline No. 33 - Long-term Consequences of Polycystic Ovary Syndrome (PDF). Unknown parameter
|origmonth=ignored (help)
Footnotes
- ↑ [Kathe] (January 16), Polycystic Ovary Syndrome (PCOS) Check
|author-link1=value (help); Check date values in:|date=, |year= / |date= mismatch(help) - ↑ Christine Cortet-Rudelli, Didier Dewailly (2006). "Diagnosis of Hyperandrogenism in Female Adolescents". Hyperandrogenism in Adolescent Girls. Armenian Health Network, Health.am. Unknown parameter
|month=ignored (help) - ↑ Pedersen SD, Brar S, Faris P, Corenblum B (2007). "Polycystic ovary syndrome: validated questionnaire for use in diagnosis". Canadian family physician Médecin de famille canadien. 53 (6): 1042–7, 1041. PMID 17872783.
- ↑ Banaszewska B, Spaczyński RZ, Pelesz M, Pawelczyk L (2003). "Incidence of elevated LH/FSH ratio in polycystic ovary syndrome women with normo- and hyperinsulinemia". Rocz. Akad. Med. Bialymst. 48: 131–4. PMID 14737959.
- ↑ Fukuoka M, Yasuda K, Fujiwara H, Kanzaki H, Mori T (1992). "Interactions between interferon gamma, tumour necrosis factor alpha, and interleukin-1 in modulating progesterone and oestradiol production by human luteinized granulosa cells in culture". Hum Reprod. 7 (10): 1361–4. PMID 1291559.
- ↑ González F, Rote N, Minium J, Kirwan J (2006). "Reactive oxygen species-induced oxidative stress in the development of insulin resistance and hyperandrogenism in polycystic ovary syndrome". J Clin Endocrinol Metab. 91 (1): 336–40. PMID 16249279.
- ↑ Legro RS, Barnhart HX, Schlaff WD (2007). "Clomiphene, Metformin, or Both for Infertility in the Polycystic Ovary Syndrome". N Engl J Med. 356 (6): 551–566. PMID 17287476.
- ↑ "Efficacy of metformin for ovulation induction in polycystic ovary syndrome". Endocrine Abstracts.
- ↑ "Diabetes Drug Helps Prevent Miscarriage". WebMD.
- ↑ "Do insulin-sensitizing drugs increase ovulation rates for women with PCOS?". Find Articles.
- ↑ Nestler J E, Jakubowicz D J, Reamer P, Gunn R D, Allan G (1999). "Ovulatory and metabolic effects of D-chiro-inositol in the polycystic ovary syndrome". N Engl J Med. 340 (17): 1314&ndash, 1320. PMID 10219066.
- ↑ Iuorno M J, Jakubowicz D J, Baillargeon J P, Dillon P, Gunn R D, Allan G, Nestler J E (2002). "Effects of d-chiro-inositol in lean women with the polycystic ovary syndrome". Endocr Pract. 8 (6): 417&ndash, 423. PMID 15251831.
- ↑ Larner J (2002). "D-chiro-inositol--its functional role in insulin action and its deficit in insulin resistance". Int J Exp Diabetes Res. 3 (1): 47&ndash, 60. PMID 11900279.
External links
Overviews
- 4women.gov Polycystic Ovarian Syndrome (PCOS)
- The University of Chicago Center for Polycystic Ovary Syndrome
- PCOS Section of The Hormone Foundation
- The Polycystic Ovary Syndrome: a starting point, not a diagnosis. and Introduction Chapter by Dr John Eden (PDF) from Polycystic Ovarian Syndrome Association of Australia POSAA
- PCOS and infertility news
- PCOS Today Magazine (based in United States) Unique perspectives about PCOS
- "Polycystic Ovary Syndrome (PCOS) FAQ". International Council on Infertility Information Dissemination. 10/9/2006. Check date values in:
|date=(help) - Project PCOS (also based in United States) Free flow of information about PCOS
- Discusses what it is like to live with PCOS
Support groups
- The Polycystic Ovarian Syndrome Association (PCOSA)
- Verity PCOS, UK support group
- SoulCysters.com, International support group
- PCOS Awareness Campaign
- PCOS in the Infertility section
- PCOStrategies
- PCOS in ConnecTion
de:Polyzystisches Ovarialsyndrom it:Sindrome dell'ovaio policistico nl:Polycysteus-ovariumsyndroom fi:Munasarjojen monirakkulaoireyhtymä sv:Polycystiskt ovariesyndrom