Plecanatide: Difference between revisions
No edit summary |
No edit summary |
||
| Line 380: | Line 380: | ||
*The responder rates are shown in Table 4. | *The responder rates are shown in Table 4. | ||
[[image:Plecanatide_Clinical_Studies_Table_2.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | [[image:Plecanatide_Clinical_Studies_Table_2.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | ||
| Line 396: | Line 390: | ||
*In Studies 3 and 4, a third randomized treatment arm of TRULANCE 6 mg once daily did not demonstrate additional treatment benefit over the 3 mg dose. Therefore, TRULANCE 6 mg once daily is not recommended. | *In Studies 3 and 4, a third randomized treatment arm of TRULANCE 6 mg once daily did not demonstrate additional treatment benefit over the 3 mg dose. Therefore, TRULANCE 6 mg once daily is not recommended. | ||
|howSupplied=*TRULANCE tablets are packaged in an aluminum foil unit dose blister pack of 30 in a child-resistant pack or in a white, opaque, high-density polyethylene round bottle with a screw-top polypropylene child-resistant cap and heat-activated induction seal. Each bottle container-closure system also contains a desiccant and a polyester coil. | |||
*TRULANCE 3 mg tablets are white to off-white, plain and round, debossed with "SP" on one side and "3" for 3 mg on the other side and supplied as: | |||
[[image:Plecanatide How Supplied Table.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | |||
|storage=*Store at room temperature, 20 to 25°C (68 to 77°F); excursions permitted to 15 to 30°C (59 to 86°F). | |storage=*Store at room temperature, 20 to 25°C (68 to 77°F); excursions permitted to 15 to 30°C (59 to 86°F). | ||
Revision as of 18:43, 20 July 2018
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Yashasvi Aryaputra[2];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
RISK OF SERIOUS DEHYDRATION IN PEDIATRIC PATIENTS
See full prescribing information for complete Boxed Warning.
|
Overview
Plecanatide is a Acetylcholine release inhibitor, Adrenergic receptor agonist that is FDA approved for the (type of indication of drug) of a list of indications, separated by commas.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include a list of adverse reactions, separated by commas..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
CONTRAINDICATIONS
Warnings
|
RISK OF SERIOUS DEHYDRATION IN PEDIATRIC PATIENTS
See full prescribing information for complete Boxed Warning.
|
Conidition 1
(Description)
Conidition 2
(Description)
Conidition 3
(Description)
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Condition 2
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
(Description)
Drug Interactions
- Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
(Description)
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Plecanatide in women who are pregnant.
Labor and Delivery
(Description)
Nursing Mothers
(Description)g
Pediatric Use
(Description)
Geriatic Use
(Description)
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
There is limited information regarding the compatibility of Plecanatide and IV administrations.
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
Plecanatide
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
(Description)
Structure
(Description with picture)
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
Chronic Idiopathic Constipation (CIC)
- The efficacy of TRULANCE for the management of symptoms of CIC was established in two 12-week, double-blind, placebo-controlled, randomized, multicenter clinical studies in adult patients (Study 1 and Study 2). In the Intention-to-Treat (ITT) population, a total of 905 patients (Study 1) and 870 patients (Study 2) were randomized 1:1 to either placebo or TRULANCE 3 mg, once daily. In clinical studies, study medication was administered without respect to food intake. Demographics for these studies included an overall mean age of 45 years (range 18 to 80 years), 80% female, 72% white, and 24% black.
- To be eligible for the studies, patients were required to meet modified Rome III criteria for at least 3 months prior to the screening visit, with symptom onset for at least 6 months prior to diagnosis. Rome III criteria were modified to require that patients report less than 3 defecations per week, rarely have a loose stool without the use of laxatives, not use manual maneuvers to facilitate defecations, and not meet criteria for IBS-C. In addition, patients were required to report at least two of the following symptoms:
- Straining during at least 25% of defecations.
- Lumpy or hard stool in at least 25% of defecations.
- Sensation of incomplete evacuations for at least 25% of defecations.
- Sensation of anorectal obstruction/blockage for at least 25% of defecations.
- Patients who met these criteria were also required to demonstrate the following during the last 2 weeks of the screening period:
- Less than 3 complete spontaneous bowel movements (CSBMs) (a CSBM is an SBM that is associated with a sense of complete evacuation) in each of the two weeks.
- Bristol Stool Form Scale (BSFS) of 6 or 7 in less than 25% of spontaneous bowel movements (SBMs) (an SBM is a bowel movement occurring in the absence of laxative use).
- One out of the following three:
- BSFS of 1 or 2 in at least 25% of defecations.
- A straining value recorded on at least 25% of days when a BM was reported.
- At least 25% of BMs result in a sense of incomplete evacuation.
- The efficacy of TRULANCE was assessed using a responder analysis and change-from-baseline in CSBM and SBM endpoints. Efficacy was assessed using information provided by patients on a daily basis in an electronic diary.
- A responder was defined as a patient who had at least 3 CSBMs in a given week and an increase of at least 1 CSBM from baseline in the same week for at least 9 weeks out of the 12 week treatment period and at least 3 of the last 4 weeks of the study. The responder rates are shown in Table 3.
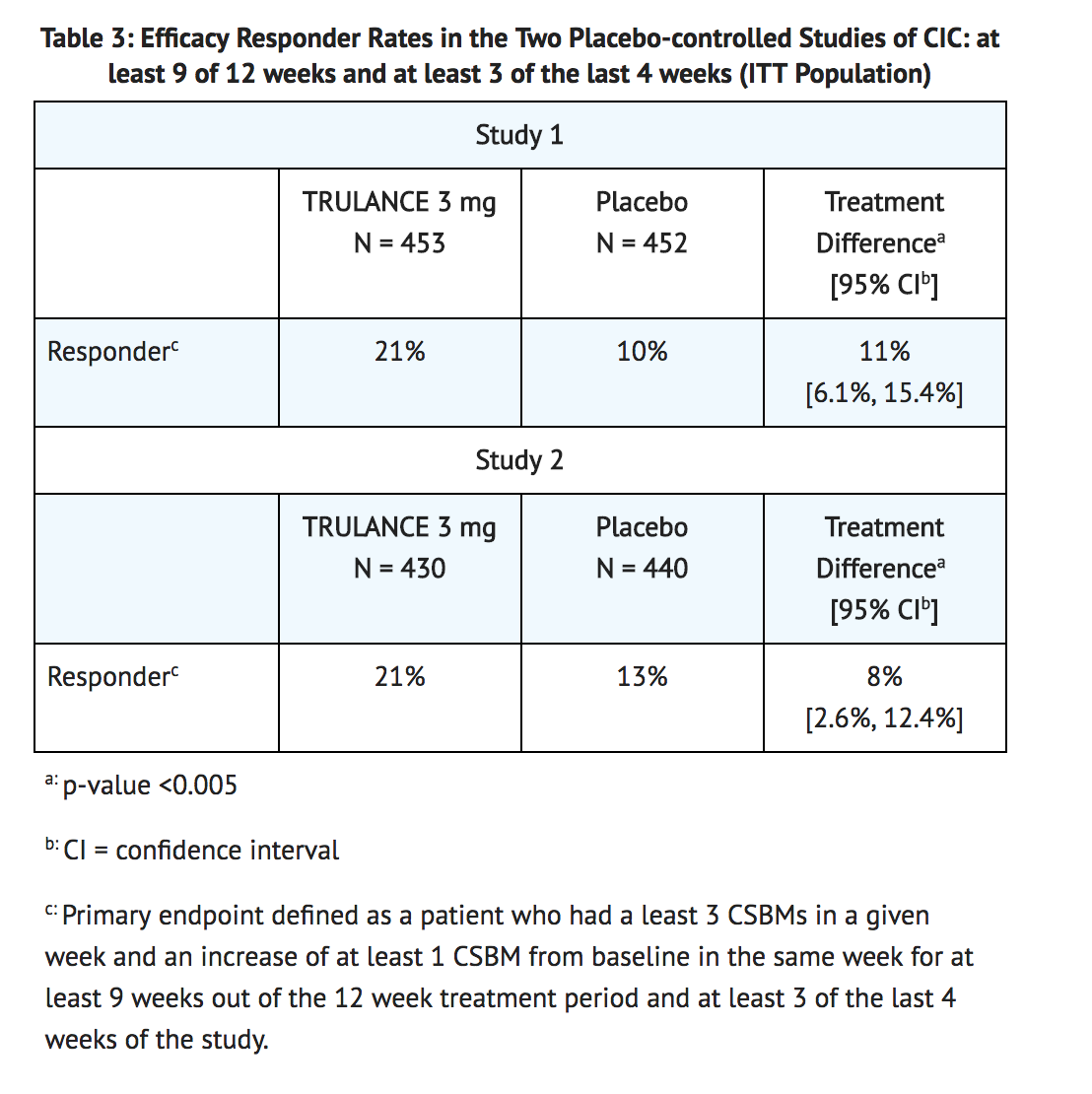
- In both studies, improvements in the frequency of CSBMs/week were seen as early as week 1 with improvement maintained through week 12. The difference between the TRULANCE group and the placebo group in the mean change of CSBMs/week frequency from baseline to week 12 was approximately 1.1 CSBMs/week.
- Over the 12 week treatment period, improvements were observed in stool frequency (number of CSBMs/week and SBMs/week) and/or stool consistency (as measured by the BSFS), and/or in the amount of straining with bowel movements (amount of time pushing or physical effort to pass stool) in the TRULANCE group as compared to placebo.
- Following completion of the study drug treatment period, patients continued to record data in the daily diary for a 2 week Post-Treatment Period. During this time, TRULANCE-treated patients generally returned to baseline for these study endpoints.
- In Studies 1 and 2, a third randomized treatment arm of TRULANCE 6 mg once daily did not demonstrate additional treatment benefit and had a greater incidence of adverse reactions than TRULANCE 3 mg once daily. Therefore, TRULANCE 6 mg once daily is not recommended.
Irritable Bowel Syndrome with Constipation (IBS-C)
- The efficacy of TRULANCE for the management of symptoms of IBS-C was established in two 12-week, double-blind, placebo-controlled, randomized, multicenter clinical studies in adult patients (Study 3 and Study 4). In the Intention-to-Treat (ITT) population, a total of 699 patients (Study 3) and 754 patients (Study 4) received treatment with placebo or TRULANCE 3 mg once daily. In clinical studies, study medication was administered without respect to food intake. Demographics for these studies included an overall mean age of 44 years (range 18 to 83 years), 74% female, 73% white, and 22% black.
- To be eligible, patients were required to meet the Rome III criteria for IBS for at least 3 months prior to the screening visit, with symptom onset for at least 6 months prior to diagnosis. Diagnosis required recurrent abdominal pain or discomfort at least 3 days/month in the last 3 months associated with 2 or more of 1) improvement with defecation, 2) onset associated with a change in frequency of stool, and 3) onset associated with a change in form (appearance) of stool. Patients also met the IBS-C differentiation criteria for constipation, characterized by a stool pattern such that at least 25% of defecations are hard or lumpy stools and no more than 25% of defecations are loose or watery stool.
- Patients who met these criteria were excluded if they demonstrated the following during the last 2 weeks of the screening period:
- Worst abdominal pain intensity (WAPI) score of 0 on an 11-point scale for more than 2 days during each week.
- An average WAPI of less than 3 for either week.
- More than 3 complete spontaneous bowel movements (CSBMs) or more than six spontaneous bowel movements (SBMs) per week in either week.
- Bristol Stool Form Scale (BSFS) of 7 for any SBM recorded.
- More than 1 day in either week with a BSFS of 6 for any SBM recorded.
- No use of rescue laxative (bisacodyl) within 72 hours before randomization.
- The efficacy of TRULANCE was assessed using a responder analysis based on abdominal pain intensity and a stool frequency responder (CSBM) endpoint. Efficacy was assessed using information provided by patients on a daily basis through an electronic phone diary system.
- A responder was defined as a patient who met both the abdominal pain intensity and stool frequency responder criteria in the same week for at least 6 of the 12 treatment weeks. The abdominal pain intensity and stool frequency responder criteria assessed each week were defined as:
- Abdominal pain intensity responder: a patient who experienced a decrease in the weekly average of worst abdominal pain in the past 24 hours score (measured daily) of at least 30% compared with baseline weekly average.
- Stool frequency responder: a patient who experienced an increase of at least 1 CSBM per week from baseline.
- The responder rates are shown in Table 4.

- In both studies, the proportion of responders who were also weekly responders for at least 2 of the 4 treatment weeks in month 3, the last month of treatment was greater in the TRULANCE groups compared to placebo.
- Over the 12 week treatment period, improvements were observed in both stool consistency (as measured by the BSFS) and in the amount of straining with bowel movements (amount of time pushing or physical effort to pass stool) in the 3 mg TRULANCE group as compared to placebo.
- Following completion of the study drug treatment period, patients continued to record data in the daily diary for a 2-week Post-Treatment Period. During this time, TRULANCE-treated patients generally returned to baseline for these study endpoints.
- In Studies 3 and 4, a third randomized treatment arm of TRULANCE 6 mg once daily did not demonstrate additional treatment benefit over the 3 mg dose. Therefore, TRULANCE 6 mg once daily is not recommended.
How Supplied
- TRULANCE tablets are packaged in an aluminum foil unit dose blister pack of 30 in a child-resistant pack or in a white, opaque, high-density polyethylene round bottle with a screw-top polypropylene child-resistant cap and heat-activated induction seal. Each bottle container-closure system also contains a desiccant and a polyester coil.
- TRULANCE 3 mg tablets are white to off-white, plain and round, debossed with "SP" on one side and "3" for 3 mg on the other side and supplied as:

Storage
- Store at room temperature, 20 to 25°C (68 to 77°F); excursions permitted to 15 to 30°C (59 to 86°F).
- Keep TRULANCE in a dry place. Protect from moisture. For bottles, keep TRULANCE in the original bottle. Do not remove desiccant from the bottle. Do not subdivide or repackage.
Images
Drug Images
{{#ask: Page Name::Plecanatide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Plecanatide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Diarrhea
- To stop TRULANCE and contact their healthcare provider if they experience severe diarrhea.
Accidental Ingestion
- Accidental ingestion of TRULANCE in children, especially in children less than 6 years of age, may result in severe diarrhea and dehydration. Instruct patients to take steps to store TRULANCE securely and out of reach of children and to dispose of unused TRULANCE.
Administration and Handling Instructions
- To take TRULANCE once daily with or without food.
- If a dose is missed, skip the missed dose and take the next dose at the regular time. Do not take two doses at the same time.
- To swallow TRULANCE tablets whole.
- If adult patients have swallowing difficulties, TRULANCE tablets can be crushed and administered orally in either applesauce or with water, or administered with water via a nasogastric or gastric feeding tube, as described in the Medication Guide.
- To keep TRULANCE in a dry place. Protect from moisture. For bottles, keep TRULANCE in the original bottle. Do not remove desiccant from the bottle. Do not subdivide or repackage. Remove and discard polyester coil after opening. Keep bottles closed tightly.

Precautions with Alcohol
Alcohol-Plecanatide interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Trulance
Look-Alike Drug Names
There is limited information regarding Plecanatide Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.