Paromomycin sulfate
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Paromomycin sulfate is a Aminoglycoside that is FDA approved for the treatment of intestinal amebiasis–acute and chronic and management of hepatic coma–as adjunctive therapy.. Common adverse reactions include Nausea, abdominal cramps, and diarrhea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Paromomycin sulfate is indicated for intestinal amebiasis–acute and chronic (NOTE-It is not effective in extraintestinal amebiasis); management of hepatic coma–as adjunctive therapy.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Paromomycin Sulfate Capsules and other antibacterial drugs, Paromomycin Sulfate Capsules should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Dosage
- Intestinal amebiasis: Adults and Pediatric Patients: Usual dose—25 to 35 mg/kg body weight daily, administered in three doses with meals, for five to ten days.
Management of hepatic coma:
Adults: Usual dose—4 g daily in divided doses, given at regular intervals for five to six days.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Paromomycin sulfate in adult patients.
Non–Guideline-Supported Use
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Indications and Dosage
- Intestinal amebiasis: Adults and Pediatric Patients: Usual dose—25 to 35 mg/kg body weight daily, administered in three doses with meals, for five to ten days.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Paromomycin sulfate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non-Guideline-Supported Use of Paromomycin sulfate in pediatric patients.
Contraindications
- Paromomycin sulfate is contraindicated in individuals with a history of previous hypersensitivity reactions to it. It is also contraindicated in intestinal obstruction.
Warnings
Precautions
- Prescribing Paromomycin Sulfate Capsules in the absence of a proven or strongly suspected bacterial infection or a prophylacticindication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
The use of this antibiotic, as with other antibiotics, may result in an overgrowth of nonsusceptible organisms, including fungi. Constant observation of the patientis essential. If new infections caused by nonsusceptible organisms appear during therapy, appropriate measures should be taken. The drug should be used with caution in individuals with ulcerative lesions of the bowel to avoid renal toxicity through inadvertent absorption.
Adverse Reactions
Clinical Trials Experience
- Nausea, abdominal cramps, and diarrhea have been reported inpatients on doses over 3 g daily.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Paromomycin sulfate in the drug label.
Drug Interactions
There is limited information regarding Paromomycin sulfate Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Paromomycin sulfate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Paromomycin sulfate during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Paromomycin sulfate with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Paromomycin sulfate with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Paromomycin sulfate with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Paromomycin sulfate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Paromomycin sulfate with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Paromomycin sulfate in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Paromomycin sulfate in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Paromomycin sulfate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Paromomycin sulfate in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Paromomycin sulfate in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Paromomycin sulfate in the drug label.
Overdosage
There is limited information regarding Overdose of Paromomycin sulfate in the drug label.
Pharmacology
There is limited information regarding Paromomycin sulfate Pharmacology in the drug label.
Mechanism of Action
- The in-vitro and in-vivo antibacterial action of paromomycin closely parallels that of neomycin.
Structure
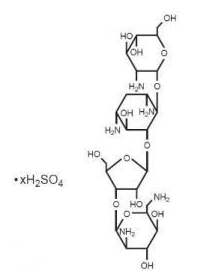
- Paromomycin sulfate is a broad spectrum antibiotic produced by Streptomyces riomosus var. paromomycinus. It is a white, amorphous, stable, water-soluble product. Paromomycin sulfate is designated chemically as 0-2, 6-Diamino-2, 6-dideoxy-β -L-idopyranosyl-(1→3)-0-β -D-ribofuranosyl-(1→5)-0-[2-amino-2-deoxy-α -D-glucopyranosyl-(1→4)]-2-deoxystreptamine sulfate (salt). The molecular formula is C23H45N5O14•xH2SO4, with a molecular weight of 615.64 (base).
Its structural formula is:
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Paromomycin sulfate in the drug label.
Pharmacokinetics
- The in-vitro and in-vivo antibacterial action of paromomycin closely parallels that of neomycin. It is poorly absorbed after oral administration, with almost 100% of the drug recoverable in the stool.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Paromomycin sulfate in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Paromomycin sulfate in the drug label.
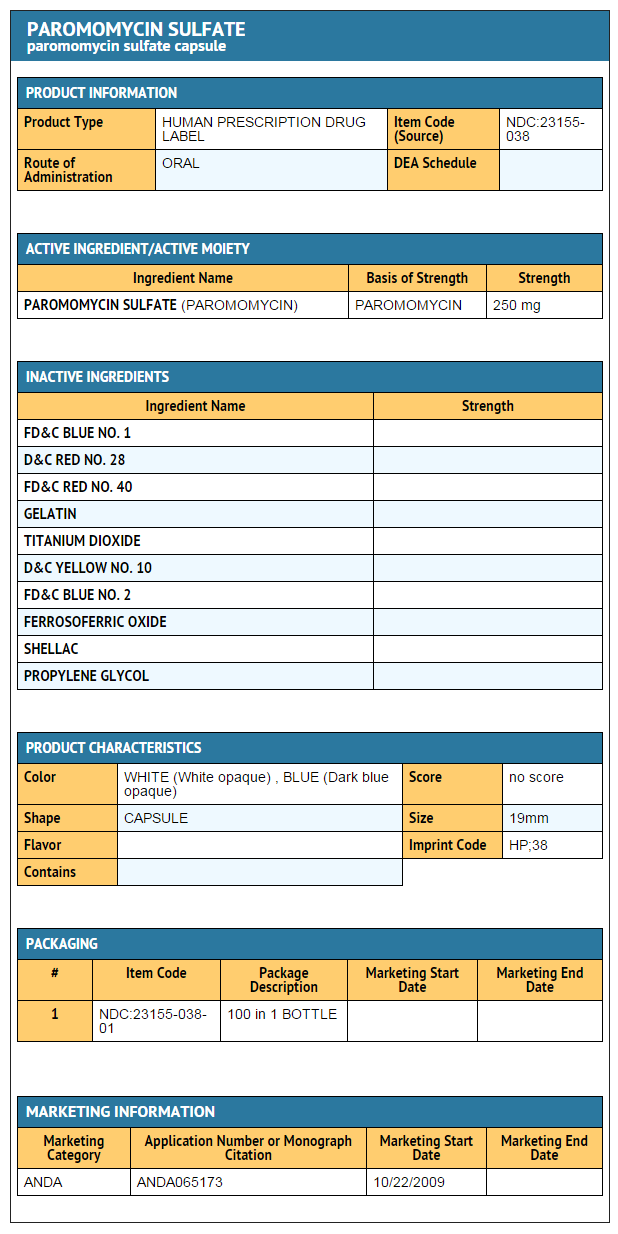
How Supplied
- Paromomycin Sulfate Capsules, USP each contain paromomycin sulfate equivalent to 250 mg paromomycin, are supplied as follows:
NDC 23155-038-01: Bottles of 100
The capsule is Dark Blue Opaque /White Opaque, imprinted with “HP 38” in black ink on the cap and on the body.
Storage
- Store at 20°-25°C (68°-77°F) [See USP controlled Room Temperature]
Protect from moisture.
Preserve in tight containers as defined in the USP.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088
Images
Drug Images
{{#ask: Page Name::Paromomycin sulfate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Paromomycin Sulfate Capsules, USP, 250 mg -100 count
Ingredients and Appearance
{{#ask: Label Page::Paromomycin sulfate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients should be counseled that antibacterial drugs including Paromomycin Sulfate Capsules should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Paromomycin Sulfate Capsules is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Paromomycin Sulfate Capsules or other antibacterial drugs in the future.
Precautions with Alcohol
- Alcohol-Paromomycin sulfate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Humatin®[3]
Look-Alike Drug Names
There is limited information regarding Paromomycin sulfate Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Flanigan TP, Ramratnam B, Graeber C, Hellinger J, Smith D, Wheeler D; et al. (1996). "Prospective trial of paromomycin for cryptosporidiosis in AIDS". Am J Med. 100 (3): 370–2. doi:10.1016/S0002-9343(97)89499-5. PMID 8629685.
- ↑ DOBON JF, CEDRATO AE, MARTINODECOLOM N (1963). "[THE USE OF PAROMOMYCIN IN INTESTINAL AMEBIASIS AND GIARDIASIS]". Dia Med. 35: 1012. PMID 14049466.
- ↑ "Paromomycin sulfate".
{{#subobject:
|Page Name=Paromomycin sulfate
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Paromomycin sulfate |Label Name=Paromomycin sulfate11.png
}}
{{#subobject:
|Label Page=Paromomycin sulfate |Label Name=Paromomycin sulfate11.png
}}