Paragonimus infection: Difference between revisions
m (Robot: Changing Category:DiseaseState to Category:Disease) |
No edit summary |
||
| Line 1: | Line 1: | ||
{{SI}} | {{SI}} | ||
{{CMG}} | {{CMG}} | ||
'''''Related Key Words and Synonyms:''''' Paragonimiasis | '''''Related Key Words and Synonyms:''''' Paragonimiasis | ||
Revision as of 02:45, 16 July 2012
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Related Key Words and Synonyms: Paragonimiasis
Epidemiology and Demographics
While P. westermani occurs in the Far East, other species of Paragonimus are encountered in Asia, the Americas, and Africa.
Pathophysiology & Etiology
Life cycle:
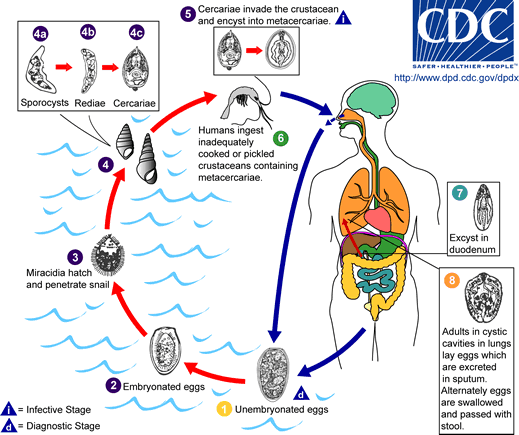
The eggs are excreted unembryonated in the sputum, or alternately they are swallowed and passed with stool 1.
In the external environment, the eggs become embryonated 2, and miracidia hatch and seek the first intermediate host, a snail, and penetrate its soft tissues 3.
Miracidia go through several developmental stages inside the snail 4: sporocysts 4a, rediae 4b, with the latter giving rise to many cercariae 4c, which emerge from the snail.
The cercariae invade the second intermediate host, a crustacean such as a crab or crayfish, where they encyst and become metacercariae. This is the infective stage for the mammalian host 5.
Human infection with P. westermani occurs by eating inadequately cooked or pickled crab or crayfish that harbor metacercariae of the parasite 6. The metacercariae excyst in the duodenum 7, penetrate through the intestinal wall into the peritoneal cavity, then through the abdominal wall and diaphragm into the lungs, where they become encapsulated and develop into adults 8 (7.5 to 12 mm by 4 to 6 mm).
The worms can also reach other organs and tissues, such as the brain and striated muscles, respectively. However, when this takes place completion of the life cycles is not achieved, because the eggs laid cannot exit these sites. Time from infection to oviposition is 65 to 90 days.
Infections may persist for 20 years in humans. Animals such as pigs, dogs, and a variety of feline species can also harbor P. westermani.
Etiologic agent:
More than 30 species of trematodes (flukes) of the genus Paragonimus have been reported which infect animals and humans. Among the more than 10 species reported to infect humans, the most common is P. westermani, the oriental lung fluke.
Diagnosis
Diagnosis is based on microscopic demonstration of eggs in stool or sputum, but these are not present until 2 to 3 months after infection. (Eggs are also occasionally encountered in effusion fluid or biopsy material.) Concentration techniques may be necessary in patients with light infections. Biopsy may allow diagnostic confirmation and species identification when an adult or developing fluke is recovered.
History and Symptoms
The acute phase (invasion and migration) may be marked by diarrhea, abdominal pain, fever, cough, urticaria, hepatosplenomegaly, pulmonary abnormalities, and eosinophilia. During the chronic phase, pulmonary manifestations include cough, expectoration of discolored sputum, hemoptysis, and chest radiographic abnormalities. Extrapulmonary locations of the adult worms result in more severe manifestations, especially when the brain is involved.
Laboratory Findings
Microscopy:
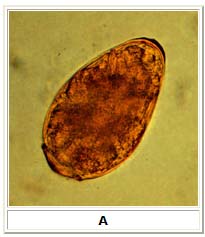
A: Egg of Paragonimus westermani. The average egg size is 85 µm by 53 µm (range: 68 to 118 µm by 39 to 67 µm). They are yellow-brown, ovoidal or elongate, with a thick shell, and often asymmetrical with one end slightly flattened. At the large end, the operculum is clearly visible. The opposite (abopercular) end is thickened. The eggs of P. westermani are excreted unembryonated.
Antibody Detection
Pulmonary paragonimiasis is the most common presentation of patients infected with Paragonimus spp., although extrapulmonary (cerebral, abdominal) paragonimiasis may occur. Detection of eggs in sputum or feces of patients with paragonimiasis is often very difficult; therefore, serodiagnosis may be very helpful in confirming infections and for monitoring the results of individual chemotherapy. In the United States, detection of antibodies to Paragonimus westermani has helped physicians differentiate paragonimiasis from tuberculosis in Indochinese immigrants. The complement fixation (CF) test has been the standard test for paragonimiasis; it is highly sensitive for diagnosis and for assessing cure after therapy. Because of the technical difficulties of CF, enzyme immunoassay (EIA) tests were developed as a replacement. The immunoblot (IB) assay performed with a crude antigen extract of P. westermani has been in use at CDC since 1988. Positive reactions, based on demonstration of an 8-kDa antigen-antibody band were obtained with serum samples of 96% of patients with parasitologically confirmed P. westermani infection. Specificity was >99%; of 210 serum specimens from patients with other parasitic and non-parasitic infections, only 1 serum sample from a patient with Schistosoma haematobium reacted. Antibody levels detected by EIA and IB do decline after chemotherapeutic cure but not as rapidly as those detected by the CF test. Most published literature deals with pulmonary paragonimiasis due to P. westermani although in some geographic areas other Paragonimus species cause similar or distinct clinical manifestations in human infections. Cross-reactivity between species does occur but at varying levels for different species. Thus, use of a test for P. westermani may not allow detection of antibodies to other Paragonimus species.
Treatment
Praziquantel is the drug of choice to treat paragonimiasis. Bithionol is an alternative drug for treatment of this disease.
References
Acknowledgements
The content on this page was first contributed by: C. Michael Gibson, M.S., M.D.