Paliperidone (injection): Difference between revisions
No edit summary |
No edit summary |
||
| Line 204: | Line 204: | ||
5.14 Disruption of Body Temperature Regulation | 5.14 Disruption of Body Temperature Regulation | ||
Disruption of the body's ability to reduce core body temperature has been attributed to antipsychotic agents. Appropriate care is advised when prescribing INVEGA® SUSTENNA® to patients who will be experiencing conditions which may contribute to an elevation in core body temperature, e.g., exercising strenuously, exposure to extreme heat, receiving concomitant medication with anticholinergic activity, or being subject to dehydration. | Disruption of the body's ability to reduce core body temperature has been attributed to antipsychotic agents. Appropriate care is advised when prescribing INVEGA® SUSTENNA® to patients who will be experiencing conditions which may contribute to an elevation in core body temperature, e.g., exercising strenuously, exposure to extreme heat, receiving concomitant medication with anticholinergic activity, or being subject to dehydration. | ||
|clinicalTrials=Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice. | |||
Commonly Reported Adverse Reactions in Double-Blind, Placebo-Controlled Clinical Trials | |||
Table 10 lists the adverse reactions reported in 2% or more of INVEGA® SUSTENNA®-treated subjects and at a greater proportion than in the placebo group with schizophrenia in the four fixed-dose, double-blind, placebo-controlled trials. | |||
[[File:INVEGA9.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
Other Adverse Reactions Observed During the Clinical Trial Evaluation of INVEGA® SUSTENNA® | |||
The following list does not include reactions: 1) already listed in previous tables or elsewhere in labeling, 2) for which a drug cause was remote, 3) which were so general as to be uninformative, or 4) which were not considered to have significant clinical implications. | |||
Cardiac disorders: atrioventricular block first degree, bradycardia, bundle branch block, palpitations, postural orthostatic tachycardia syndrome, tachycardia | |||
Ear and labyrinth disorders: vertigo | |||
Eye disorders: eye movement disorder, eye rolling, oculogyric crisis, vision blurred | |||
Gastrointestinal disorders: constipation, dyspepsia, flatulence, salivary hypersecretion | |||
Immune system disorders: hypersensitivity | |||
Investigations: alanine aminotransferase increased, aspartate aminotransferase increased, electrocardiogram abnormal | |||
Metabolism and nutrition disorders: decreased appetite, hyperinsulinemia, increased appetite | |||
Musculoskeletal and connective tissue disorders: arthralgia, joint stiffness, muscle rigidity, muscle spasms, muscle tightness, muscle twitching, nuchal rigidity | |||
Nervous system disorders: bradykinesia, cerebrovascular accident, convulsion, dizziness postural, drooling, dysarthria, dyskinesia, dystonia, hypertonia, lethargy, oromandibular dystonia, parkinsonism, psychomotor hyperactivity, syncope | |||
Psychiatric disorders: insomnia, restlessness | |||
Reproductive system and breast disorders: amenorrhea, breast discharge, erectile dysfunction, galactorrhea, gynecomastia, menstrual disorder, menstruation delayed, menstruation irregular, sexual dysfunction | |||
Respiratory, thoracic and mediastinal disorders: nasal congestion | |||
Skin and subcutaneous tissue disorders: drug eruption, pruritus, pruritus generalized, rash, urticaria | |||
Discontinuations Due to Adverse Events | |||
The percentage of subjects who discontinued due to adverse events in the four fixed-dose, double-blind, placebo-controlled schizophrenia trials were similar for INVEGA® SUSTENNA®- and placebo-treated subjects. | |||
The percentage of subjects who discontinued due to adverse events in the open-label period of the long-term study in subjects with schizoaffective disorder was 7.5%. During the double-blind, placebo-controlled period of that study, the percentages of subjects who discontinued due to adverse events were 5.5% and 1.8% in INVEGA® SUSTENNA®- and placebo-treated subjects, respectively. | |||
Dose-Related Adverse Reactions | |||
Based on the pooled data from the four fixed-dose, double-blind, placebo-controlled trials in subjects with schizophrenia, among the adverse reactions that occurred at ≥ 2% incidence in the subjects treated with INVEGA® SUSTENNA®, only akathisia increased with dose. Hyperprolactinemia also exhibited a dose relationship, but did not occur at ≥ 2% incidence in INVEGA® SUSTENNA®-treated subjects from the four fixed-dose studies. | |||
Demographic Differences | |||
An examination of population subgroups in the double-blind placebo-controlled trials did not reveal any evidence of differences in safety on the basis of age, gender, or race alone; however, there were few subjects ≥ 65 years of age. | |||
Extrapyramidal Symptoms (EPS) | |||
Pooled data from the two double-blind, placebo-controlled, 13-week, fixed-dose trials in adult subjects with schizophrenia provided information regarding EPS. Several methods were used to measure EPS: (1) the Simpson-Angus global score (mean change from baseline or score at the end of trial) which broadly evaluates Parkinsonism, (2) the Barnes Akathisia Rating Scale global clinical rating score (mean change from baseline or score at the end of trial) which evaluates akathisia, (3) use of anticholinergic medications to treat EPS, (4) the Abnormal Involuntary Movement Scale scores (mean change from baseline or scores at the end of trial) (Table 11), and (5) incidence of spontaneous reports of EPS (Table 12). | |||
[[File:INVEGA10.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
[[File:INVEGA11.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
The results across all phases of the maintenance trial in subjects with schizophrenia exhibited comparable findings. In the 9-week, fixed-dose, double-blind, placebo-controlled trial, the proportions of Parkinsonism and akathisia assessed by incidence of rating scales were higher in the INVEGA® SUSTENNA® 156 mg group (18% and 11%, respectively) than in the INVEGA® SUSTENNA® 78 mg group (9% and 5%, respectively) and placebo group (7% and 4%, respectively). | |||
In the 13-week study in subjects with schizophrenia involving 234 mg initiation dosing, the incidence of any EPS was similar to that of the placebo group (8%), but exhibited a dose-related pattern with 6%, 10%, and 11% in the INVEGA® SUSTENNA® 234/39 mg, 234/156 mg, and 234/234 mg groups, respectively. Hyperkinesia was the most frequent category of EPS-related adverse events in this study, and was reported at a similar rate between the placebo (4.9%) and INVEGA® SUSTENNA® 234/156 mg (4.8%) and 234/234 mg (5.5%) groups, but at a lower rate in the 234/39 mg group (1.3%). | |||
In the long-term study in subjects with schizoaffective disorder, the EPS during the 25-week open-label INVEGA® SUSTENNA® treatment were hyperkinesia (12.3%), parkinsonism (8.7%), tremor (3.4%), dyskinesia (2.5%), and dystonia (2.1%). During the 15-month double-blind treatment, the incidence of any EPS was similar to that of the placebo group (8.5% and 7.1% respectively). The most commonly reported treatment-emergent EPS-related adverse events (>2%) in any treatment group in the double-blind phase of the study (INVEGA® SUSTENNA® versus placebo) were hyperkinesia (3.7% vs. 2.9%), parkinsonism (3.0% vs. 1.8%), and tremor (1.2% vs. 2.4%). | |||
Dystonia | |||
Symptoms of dystonia, prolonged abnormal contractions of muscle groups, may occur in susceptible individuals during the first few days of treatment. Dystonic symptoms include: spasm of the neck muscles, sometimes progressing to tightness of the throat, swallowing difficulty, difficulty breathing, and/or protrusion of the tongue. While these symptoms can occur at low doses, they occur more frequently and with greater severity with high potency and at higher doses of first generation antipsychotic drugs. An elevated risk of acute dystonia is observed in males and younger age groups. | |||
Laboratory Test Abnormalities | |||
In the pooled data from the two double-blind, placebo-controlled, 13-week, fixed-dose trials in subjects with schizophrenia, a between-group comparison revealed no medically important differences between INVEGA® SUSTENNA® and placebo in the proportions of subjects experiencing potentially clinically significant changes in routine serum chemistry, hematology, or urinalysis parameters. Similarly, there were no differences between INVEGA® SUSTENNA® and placebo in the incidence of discontinuations due to changes in hematology, urinalysis, or serum chemistry, including mean changes from baseline in fasting glucose, insulin, c-peptide, triglycerides, HDL, LDL, and total cholesterol measurements. However, INVEGA® SUSTENNA® was associated with increases in serum prolactin [see WARNINGS AND PRECAUTIONS (5.9)]. The results from the 13-week study involving 234 mg initiation dosing, the 9-week, fixed-dose, double-blind, placebo-controlled trial, and the double-blind phase of the maintenance trial in subjects with schizophrenia exhibited comparable findings. | |||
Pain Assessment and Local Injection Site Reactions | |||
In the pooled data from the two 13-week, fixed-dose, double-blind, placebo-controlled trials in subjects with schizophrenia, the mean intensity of injection pain reported by subjects using a visual analog scale (0 = no pain to 100 = unbearably painful) decreased in all treatment groups from the first to the last injection (placebo: 10.9 to 9.8; 39 mg: 10.3 to 7.7; 78 mg: 10.0 to 9.2; 156 mg: 11.1 to 8.8). The results from both the 9-week, fixed-dose, double-blind, placebo-controlled trial and the double-blind phase of the maintenance trial exhibited comparable findings. | |||
In the 13-week study involving 234 mg initiation dosing in subjects with schizophrenia, occurrences of induration, redness, or swelling, as assessed by blinded study personnel, were infrequent, generally mild, decreased over time, and similar in incidence between the INVEGA® SUSTENNA® and placebo groups. Investigator ratings of injection pain were similar for the placebo and INVEGA® SUSTENNA® groups. Investigator evaluations of the injection site after the first injection for redness, swelling, induration, and pain were rated as absent for 69–100% of subjects in both the INVEGA® SUSTENNA® and placebo groups. At Day 92, investigators rated absence of redness, swelling, induration, and pain in 95–100% of subjects in both the INVEGA® SUSTENNA® and placebo groups. | |||
Adverse Reactions Reported in Clinical Trials with Oral Paliperidone | |||
The following is a list of additional adverse reactions that have been reported in clinical trials with oral paliperidone: | |||
Cardiac disorders: bundle branch block left, sinus arrhythmia | |||
Gastrointestinal disorders: abdominal pain, small intestinal obstruction | |||
General disorders and administration site conditions: edema, edema peripheral | |||
Immune system disorders: anaphylactic reaction | |||
Infections and infestations: rhinitis | |||
Musculoskeletal and connective tissue disorders: musculoskeletal pain, torticollis, trismus | |||
Nervous system disorders: cogwheel rigidity, grand mal convulsion, parkinsonian gait, transient ischemic attack | |||
Psychiatric disorders: sleep disorder | |||
Reproductive system and breast disorders: breast engorgement, breast tenderness/breast pain, retrograde ejaculation | |||
Respiratory, thoracic and mediastinal disorders: pharyngolaryngeal pain, pneumonia aspiration | |||
Skin and subcutaneous tissue disorders: rash papular | |||
Vascular disorders: hypotension, ischemia | |||
6.2 Postmarketing Experience | |||
The following adverse reactions have been identified during postapproval use of paliperidone; because these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Reactions already listed in other parts of ADVERSE REACTIONS (6), or those considered in WARNINGS AND PRECAUTIONS (5) are not listed here. | |||
Blood disorders: thrombotic thrombocytopenic purpura | |||
Gastrointestinal disorders: ileus | |||
Genitourinary disorders: urinary incontinence, urinary retention | |||
Immune system disorders: angioedema, swollen tongue | |||
Cases of anaphylactic reaction after injection with INVEGA® SUSTENNA® have been reported during postmarketing experience in patients who have previously tolerated oral risperidone or oral paliperidone. | |||
6.3 Adverse Reactions Reported With Risperidone | |||
Paliperidone is the major active metabolite of risperidone. Adverse reactions reported with oral risperidone and risperidone long-acting injection can be found in the ADVERSE REACTIONS sections of the package inserts for those products. | |||
|drugInteractions=Because paliperidone palmitate is hydrolyzed to paliperidone [see CLINICAL PHARMACOLOGY (12.3)], results from studies with oral paliperidone should be taken into consideration when assessing drug-drug interaction potential. | |||
7.1 Potential for INVEGA® SUSTENNA® to Affect Other Drugs | |||
Paliperidone may antagonize the effect of levodopa and other dopamine agonists. | |||
Because of its potential for inducing orthostatic hypotension, an additive effect may occur when INVEGA® SUSTENNA® is administered with other therapeutic agents that have this potential [see WARNINGS AND PRECAUTIONS (5.7)]. | |||
No dose adjustment is necessary for lithium when it is coadministered with INVEGA® SUSTENNA®. Pharmacokinetic interaction between INVEGA® SUSTENNA® and lithium is unlikely. | |||
No dose adjustment is necessary for valproate when INVEGA® SUSTENNA® is added to the therapy. Steady-state pharmacokinetics of valproate was not affected when patients were coadministered oral paliperidone extended-release tablets [see CLINICAL PHARMACOLOGY (12.3)]. | |||
Paliperidone is not expected to cause clinically important pharmacokinetic interactions with drugs that are metabolized by cytochrome P450 isozymes [see CLINICAL PHARMACOLOGY (12.3)]. | |||
7.2 Potential for Other Drugs to Affect INVEGA® SUSTENNA® | |||
On initiation of strong inducers of both CYP3A4 and P-gp (e.g., carbamazepine, rifampin, or St John's wort), it may be necessary to increase the dose of INVEGA® SUSTENNA®. Conversely, on discontinuation of the strong inducer, it may be necessary to decrease the dose of INVEGA® SUSTENNA® [see CLINICAL PHARMACOLOGY (12.3)]. | |||
No dose adjustment is necessary for INVEGA® SUSTENNA® when valproate is added to treatment [see CLINICAL PHARMACOLOGY (12.3)]. | |||
No dose adjustment is necessary for INVEGA® SUSTENNA® when it is coadministered with lithium. Pharmacokinetic interaction between INVEGA® SUSTENNA® and lithium is unlikely. | |||
In vitro studies indicate that CYP2D6 and CYP3A4 may be involved in paliperidone metabolism; however, there is no evidence in vivo that inhibitors of these enzymes significantly affect the metabolism of paliperidone. Paliperidone is not a substrate of CYP1A2, CYP2A6, CYP2C9, and CYP2C19; an interaction with inhibitors or inducers of these isozymes is unlikely. | |||
|alcohol=Alcohol-Paliperidone (injection) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Paliperidone (injection) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | }} | ||
Revision as of 20:33, 19 May 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Turky Alkathery, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
See full prescribing information for complete Boxed Warning.
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death [see WARNINGS AND PRECAUTIONS (5.1)].
INVEGA® SUSTENNA® is not approved for use in patients with dementia-related psychosis [see WARNINGS AND PRECAUTIONS (5.1)].
|
Overview
Paliperidone (injection) is an atypical antipsychotic that is FDA approved for the treatment of schizophrenia. There is a Black Box Warning for this drug as shown here. Common adverse reactions include injection site reactions, somnolence/sedation, dizziness, akathisia, and extrapyramidal disorder.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
INVEGA® SUSTENNA® (paliperidone palmitate) is indicated for the treatment of:
Schizophrenia [see CLINICAL STUDIES 14.1]. Schizoaffective disorder as monotherapy and as an adjunct to mood stabilizers or antidepressants [see CLINICAL STUDIES 14.2].
Dosage
2.1 Administration Instructions Each injection must be administered only by a health care professional.
Parenteral drug products should be inspected visually for foreign matter and discoloration prior to administration, whenever product and container permit.
INVEGA® SUSTENNA® is intended for intramuscular use only. Do not administer by any other route. Avoid inadvertent injection into a blood vessel. Administer the dose in a single injection; do not administer the dose in divided injections. Inject slowly, deep into the muscle.
The recommended needle size for administration of INVEGA® SUSTENNA® into the deltoid muscle is determined by the patient's weight:
For patients weighing less than 90 kg, the 1-inch, 23 gauge needle is recommended. For patients weighing 90 kg or more, the 1½-inch, 22 gauge needle is recommended. Deltoid injections should be alternated between the two deltoid muscles.
The recommended needle size for administration of INVEGA® SUSTENNA® into the gluteal muscle is the 1½-inch, 22 gauge needle regardless of patient weight.
Administer into the upper-outer quadrant of the gluteal muscle. Gluteal injections should be alternated between the two gluteal muscles.
2.2 Schizophrenia and Schizoaffective Disorder For patients who have never taken oral paliperidone or oral or injectable risperidone, it is recommended to establish tolerability with oral paliperidone or oral risperidone prior to initiating treatment with INVEGA® SUSTENNA®.
The recommended dosing of INVEGA® SUSTENNA® for each approved indication is displayed in Table 1. The recommended initiation of INVEGA® SUSTENNA® is with a dose of 234 mg on treatment day 1 and 156 mg one week later, both administered in the deltoid muscle. Following the second initiation dose, monthly maintenance doses can be administered in either the deltoid or gluteal muscle.
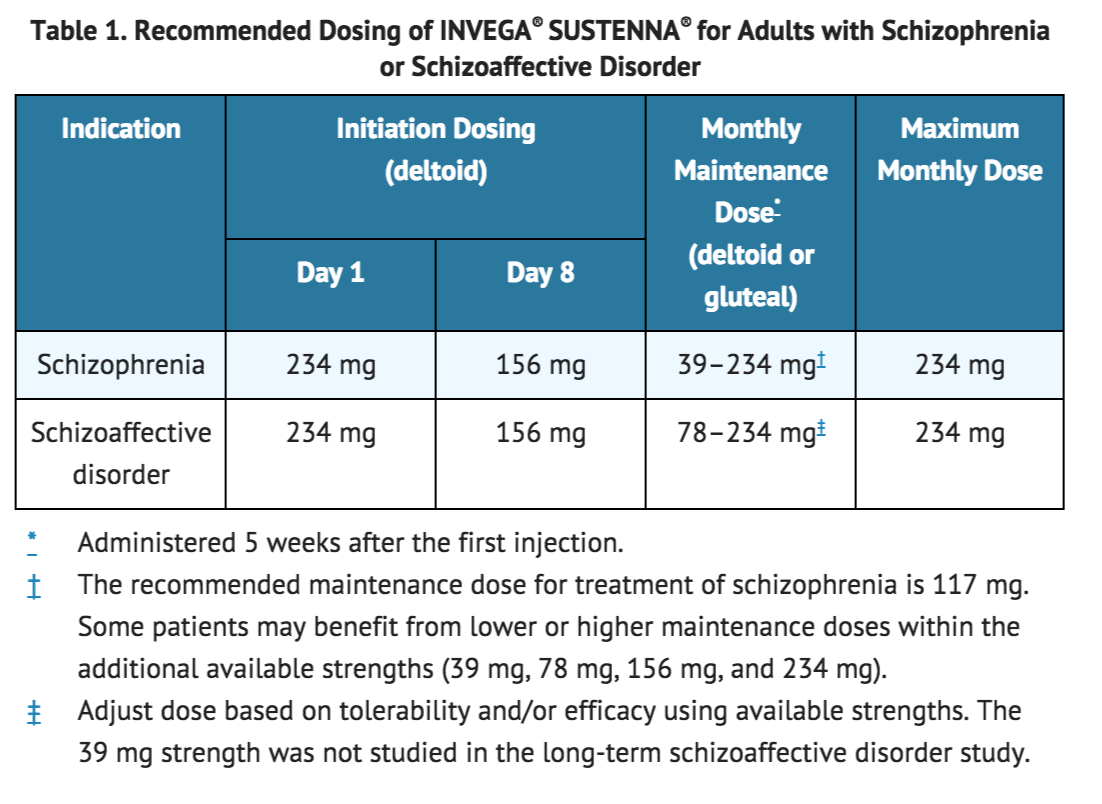
Adjustment of the maintenance dose may be made monthly. When making dose adjustments, the prolonged-release characteristics of INVEGA® SUSTENNA® should be considered [see CLINICAL PHARMACOLOGY (12.3)], as the full effect of the dose adjustment may not be evident for several months.
2.3 Missed Doses Avoiding Missed Doses
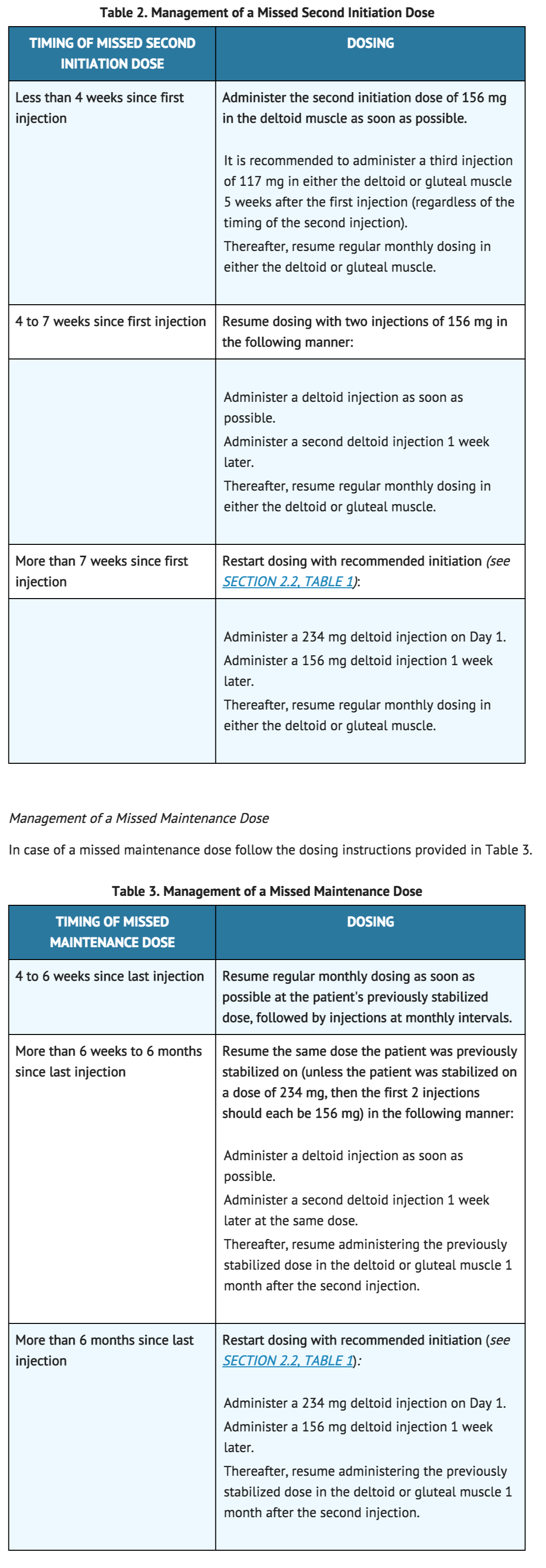
It is recommended that the second initiation dose of INVEGA® SUSTENNA® be given one week after the first dose. To avoid a missed dose, patients may be given the second dose 4 days before or after the one-week time point. Similarly, the third and subsequent injections after the initiation regimen are recommended to be given monthly. To avoid a missed monthly dose, patients may be given the injection up to 7 days before or after the monthly time point.
Management of a Missed Second Initiation Dose
If the target date for the second INVEGA® SUSTENNA® injection (one week ± 4 days) is missed, the recommended reinitiation depends on the length of time which has elapsed since the patient's first injection. In case of a missed second initiation dose follow the dosing instructions provided in Table 2.
2.4 Use with Oral Paliperidone or with Risperidone Concomitant use of INVEGA® SUSTENNA® with oral paliperidone or oral or injectable risperidone has not been studied. Since paliperidone is the major active metabolite of risperidone, consideration should be given to the additive paliperidone exposure if any of these medications are coadministered with INVEGA® SUSTENNA®.
2.5 Dosage Adjustments Renal Impairment
INVEGA® SUSTENNA® has not been systematically studied in patients with renal impairment [see CLINICAL PHARMACOLOGY (12.3)]. For patients with mild renal impairment (creatinine clearance ≥ 50 mL/min to < 80 mL/min [Cockcroft-Gault Formula]), initiate INVEGA® SUSTENNA® with a dose of 156 mg on treatment day 1 and 117 mg one week later. Administer both doses in the deltoid muscle. Thereafter, follow with monthly injections of 78 mg in either the deltoid or gluteal muscle [see USE IN SPECIFIC POPULATIONS (8.6) and CLINICAL PHARMACOLOGY (12.3)].
INVEGA® SUSTENNA® is not recommended in patients with moderate or severe renal impairment (creatinine clearance < 50 mL/min) [see USE IN SPECIFIC POPULATIONS (8.6) and CLINICAL PHARMACOLOGY (12.3)].
Coadministration with Strong CYP3A4/P-glycoprotein (P-gp) Inducers
It may be necessary to increase the dose of INVEGA® SUSTENNA® when a strong inducer of both CYP3A4 and P-gp (e.g., carbamazepine, rifampin, St John's wort) is co-administered. Conversely, on discontinuation of the strong inducer, it may be necessary to decrease the dose of INVEGA® SUSTENNA® [see DRUG INTERACTIONS (7.2) and CLINICAL PHARMACOLOGY (12.3)].
2.6 Switching from Other Antipsychotics There are no systematically collected data to specifically address switching patients with schizophrenia or schizoaffective disorder from other antipsychotics to INVEGA® SUSTENNA®, or concerning concomitant administration with other antipsychotics.
2.6.1 Switching from Oral Antipsychotics
For patients who have never taken oral paliperidone or oral or injectable risperidone, tolerability should be established with oral paliperidone or oral risperidone prior to initiating treatment with INVEGA® SUSTENNA®.
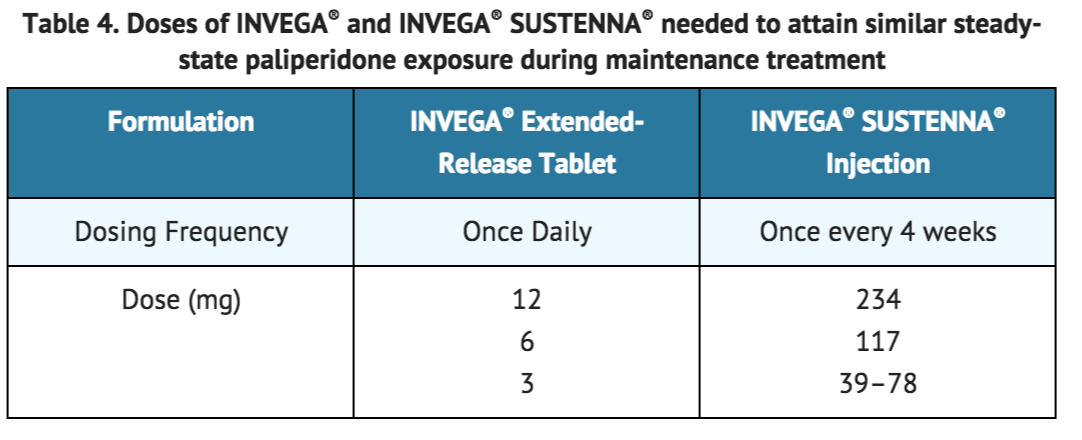
Previous oral antipsychotics can be discontinued at the time of initiation of treatment with INVEGA® SUSTENNA®. Recommended initiation of INVEGA® SUSTENNA® is with a dose of 234 mg on treatment day 1 and 156 mg one week later, both administered in the deltoid muscle [see DOSAGE AND ADMINISTRATION (2.2)]. Patients previously stabilized on different doses of INVEGA® Extended-Release tablets can attain similar paliperidone steady-state exposure during maintenance treatment with INVEGA® SUSTENNA® monthly doses as depicted in Table 4.
2.6.2 Switching from Long-Acting Injectable Antipsychotics
For patients who have never taken oral paliperidone or oral or injectable risperidone, tolerability should be established with oral paliperidone or oral risperidone prior to initiating treatment with INVEGA® SUSTENNA®.
When switching patients currently at steady-state on a long-acting injectable antipsychotic, initiate INVEGA® SUSTENNA® therapy in place of the next scheduled injection. INVEGA® SUSTENNA® should then be continued at monthly intervals. The one-week initiation dosing regimen as described in Section 2.2 is not required. See TABLE 1 above for recommended monthly maintenance dosing. Based on previous clinical history of tolerability and/or efficacy, some patients may benefit from lower or higher maintenance doses within the available strengths (39 mg, 78 mg, 117 mg, 156 mg, and 234 mg). The 39 mg strength was not studied in the long-term schizoaffective disorder study. Monthly maintenance doses can be administered in either the deltoid or gluteal muscle [see DOSAGE AND ADMINISTRATION (2.2)].
If INVEGA® SUSTENNA® is discontinued, its prolonged-release characteristics must be considered. As recommended with other antipsychotic medications, the need for continuing existing extrapyramidal symptoms (EPS) medication should be re-evaluated periodically.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Paliperidone (injection) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Paliperidone (injection) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Paliperidone (injection) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Paliperidone (injection) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Paliperidone (injection) in pediatric patients.
Contraindications
INVEGA® SUSTENNA® is contraindicated in patients with a known hypersensitivity to either paliperidone or risperidone, or to any of the excipients in the INVEGA® SUSTENNA® formulation. Hypersensitivity reactions, including anaphylactic reactions and angioedema, have been observed in patients treated with risperidone and paliperidone. Paliperidone palmitate is converted to paliperidone, which is a metabolite of risperidone.
Warnings
|
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
See full prescribing information for complete Boxed Warning.
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death [see WARNINGS AND PRECAUTIONS (5.1)].
INVEGA® SUSTENNA® is not approved for use in patients with dementia-related psychosis [see WARNINGS AND PRECAUTIONS (5.1)].
|
5.1 Increased Mortality in Elderly Patients with Dementia-Related Psychosis Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of 17 placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. INVEGA® SUSTENNA® (paliperidone palmitate) is not approved for the treatment of patients with dementia-related psychosis [see BOXED WARNING].
5.2 Cerebrovascular Adverse Reactions, Including Stroke, in Elderly Patients with Dementia-Related Psychosis In placebo-controlled trials with risperidone, aripiprazole, and olanzapine in elderly subjects with dementia, there was a higher incidence of cerebrovascular adverse reactions (cerebrovascular accidents and transient ischemic attacks) including fatalities compared to placebo-treated subjects. Oral paliperidone and INVEGA® SUSTENNA® were not marketed at the time these studies were performed and are not approved for the treatment of patients with dementia-related psychosis [see BOXED WARNING and WARNINGS AND PRECAUTIONS (5.1)].
5.3 Neuroleptic Malignant Syndrome A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with antipsychotic drugs, including INVEGA® SUSTENNA®.
Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmia). Additional signs may include elevated creatine phosphokinase, myoglobinuria (rhabdomyolysis), and acute renal failure.
The diagnostic evaluation of patients with this syndrome is complicated. In arriving at a diagnosis, it is important to identify cases in which the clinical presentation includes both serious medical illness (e.g., pneumonia, systemic infection, etc.) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever, and primary central nervous system pathology.
The management of NMS should include: (1) immediate discontinuation of antipsychotic drugs and other drugs not essential to concurrent therapy; (2) intensive symptomatic treatment and medical monitoring; and (3) treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for uncomplicated NMS.
If a patient appears to require antipsychotic drug treatment after recovery from NMS, reintroduction of drug therapy should be closely monitored, since recurrences of NMS have been reported.
5.4 QT Prolongation Paliperidone causes a modest increase in the corrected QT (QTc) interval. The use of paliperidone should be avoided in combination with other drugs that are known to prolong QTc including Class 1A (e.g., quinidine, procainamide) or Class III (e.g., amiodarone, sotalol) antiarrhythmic medications, antipsychotic medications (e.g., chlorpromazine, thioridazine), antibiotics (e.g., gatifloxacin, moxifloxacin), or any other class of medications known to prolong the QTc interval. Paliperidone should also be avoided in patients with congenital long QT syndrome and in patients with a history of cardiac arrhythmias.
Certain circumstances may increase the risk of the occurrence of Torsades de pointes and/or sudden death in association with the use of drugs that prolong the QTc interval, including (1) bradycardia; (2) hypokalemia or hypomagnesemia; (3) concomitant use of other drugs that prolong the QTc interval; and (4) presence of congenital prolongation of the QT interval.
The effects of oral paliperidone on the QT interval were evaluated in a double-blind, active-controlled (moxifloxacin 400 mg single dose), multicenter QT study in adults with schizophrenia and schizoaffective disorder, and in three placebo- and active-controlled 6-week, fixed-dose efficacy trials in adults with schizophrenia.
In the QT study (n=141), the 8 mg dose of immediate-release oral paliperidone (n=50) showed a mean placebo-subtracted increase from baseline in QTcLD of 12.3 msec (90% CI: 8.9; 15.6) on day 8 at 1.5 hours post-dose. The mean steady-state peak plasma concentration for this 8 mg dose of paliperidone immediate release (Cmax ss = 113 ng/mL) was more than 2-fold the exposure observed with the maximum recommended 234 mg dose of INVEGA® SUSTENNA® administered in the deltoid muscle (predicted median Cmax ss = 50 ng/mL). In this same study, a 4 mg dose of the immediate-release oral formulation of paliperidone, for which Cmax ss = 35 ng/mL, showed an increased placebo-subtracted QTcLD of 6.8 msec (90% CI: 3.6; 10.1) on day 2 at 1.5 hours post-dose.
In the three fixed-dose efficacy studies of oral paliperidone extended release in subjects with schizophrenia, electrocardiogram (ECG) measurements taken at various time points showed only one subject in the oral paliperidone 12 mg group had a change exceeding 60 msec at one time-point on Day 6 (increase of 62 msec).
In the four fixed-dose efficacy studies of INVEGA® SUSTENNA® in subjects with schizophrenia and in the long-term study in subjects with schizoaffective disorder, no subject experienced a change in QTcLD exceeding 60 msec and no subject had a QTcLD value of > 500 msec at any time point. In the maintenance study in subjects with schizophrenia, no subject had a QTcLD change > 60 msec, and one subject had a QTcLD value of 507 msec (Bazett's QT corrected interval [QTcB] value of 483 msec); this latter subject also had a heart rate of 45 beats per minute.
5.5 Tardive Dyskinesia A syndrome of potentially irreversible, involuntary, dyskinetic movements may develop in patients treated with antipsychotic drugs. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to predict which patients will develop the syndrome. Whether antipsychotic drug products differ in their potential to cause tardive dyskinesia is unknown.
The risk of developing tardive dyskinesia and the likelihood that it will become irreversible appear to increase as the duration of treatment and the total cumulative dose of antipsychotic drugs administered to the patient increase, but the syndrome can develop after relatively brief treatment periods at low doses, although this is uncommon.
There is no known treatment for established tardive dyskinesia, although the syndrome may remit, partially or completely, if antipsychotic treatment is withdrawn. Antipsychotic treatment itself may suppress (or partially suppress) the signs and symptoms of the syndrome and may thus mask the underlying process. The effect of symptomatic suppression on the long-term course of the syndrome is unknown.
Given these considerations, INVEGA® SUSTENNA® should be prescribed in a manner that is most likely to minimize the occurrence of tardive dyskinesia. Chronic antipsychotic treatment should generally be reserved for patients who suffer from a chronic illness that is known to respond to antipsychotic drugs. In patients who do require chronic treatment, the smallest dose and the shortest duration of treatment producing a satisfactory clinical response should be sought. The need for continued treatment should be reassessed periodically.
If signs and symptoms of tardive dyskinesia appear in a patient treated with INVEGA® SUSTENNA®, drug discontinuation should be considered. However, some patients may require treatment with INVEGA® SUSTENNA® despite the presence of the syndrome.
5.6 Metabolic Changes Atypical antipsychotic drugs have been associated with metabolic changes that may increase cardiovascular/cerebrovascular risk. These metabolic changes include hyperglycemia, dyslipidemia, and body weight gain. While all of the drugs in the class have been shown to produce some metabolic changes, each drug has its own specific risk profile.
Hyperglycemia and Diabetes Mellitus
Hyperglycemia and diabetes mellitus, in some cases extreme and associated with ketoacidosis or hyperosmolar coma or death, have been reported in patients treated with all atypical antipsychotics. These cases were, for the most part, seen in post-marketing clinical use and epidemiologic studies, not in clinical trials, and there have been few reports of hyperglycemia or diabetes in trial subjects treated with INVEGA® SUSTENNA®. Assessment of the relationship between atypical antipsychotic use and glucose abnormalities is complicated by the possibility of an increased background risk of diabetes mellitus in patients with schizophrenia and the increasing incidence of diabetes mellitus in the general population. Given these confounders, the relationship between atypical antipsychotic use and hyperglycemia-related adverse reactions is not completely understood. However, epidemiological studies suggest an increased risk of hyperglycemia-related adverse reactions in patients treated with the atypical antipsychotics. Because INVEGA® SUSTENNA® was not marketed at the time these studies were performed, it is not known if INVEGA® SUSTENNA® is associated with this risk.
Patients with an established diagnosis of diabetes mellitus who are started on atypical antipsychotics should be monitored regularly for worsening of glucose control. Patients with risk factors for diabetes mellitus (e.g., obesity, family history of diabetes) who are starting treatment with atypical antipsychotics should undergo fasting blood glucose testing at the beginning of treatment and periodically during treatment. Any patient treated with atypical antipsychotics should be monitored for symptoms of hyperglycemia including polydipsia, polyuria, polyphagia, and weakness. Patients who develop symptoms of hyperglycemia during treatment with atypical antipsychotics should undergo fasting blood glucose testing. In some cases, hyperglycemia has resolved when the atypical antipsychotic was discontinued; however, some patients required continuation of anti-diabetic treatment despite discontinuation of the suspect drug.
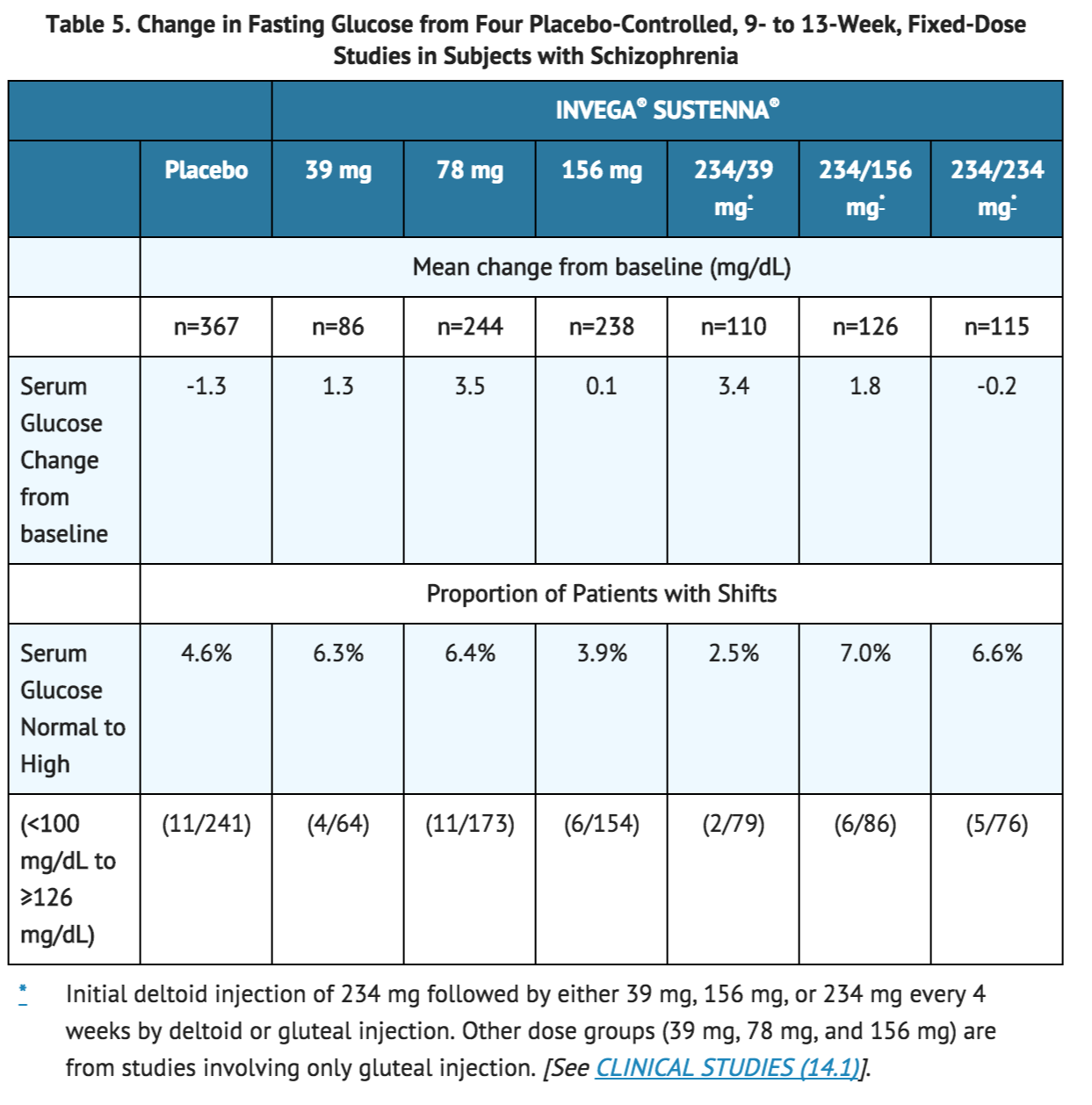
Pooled data from the four placebo-controlled (one 9-week and three 13-week), fixed-dose studies in subjects with schizophrenia are presented in Table 5.
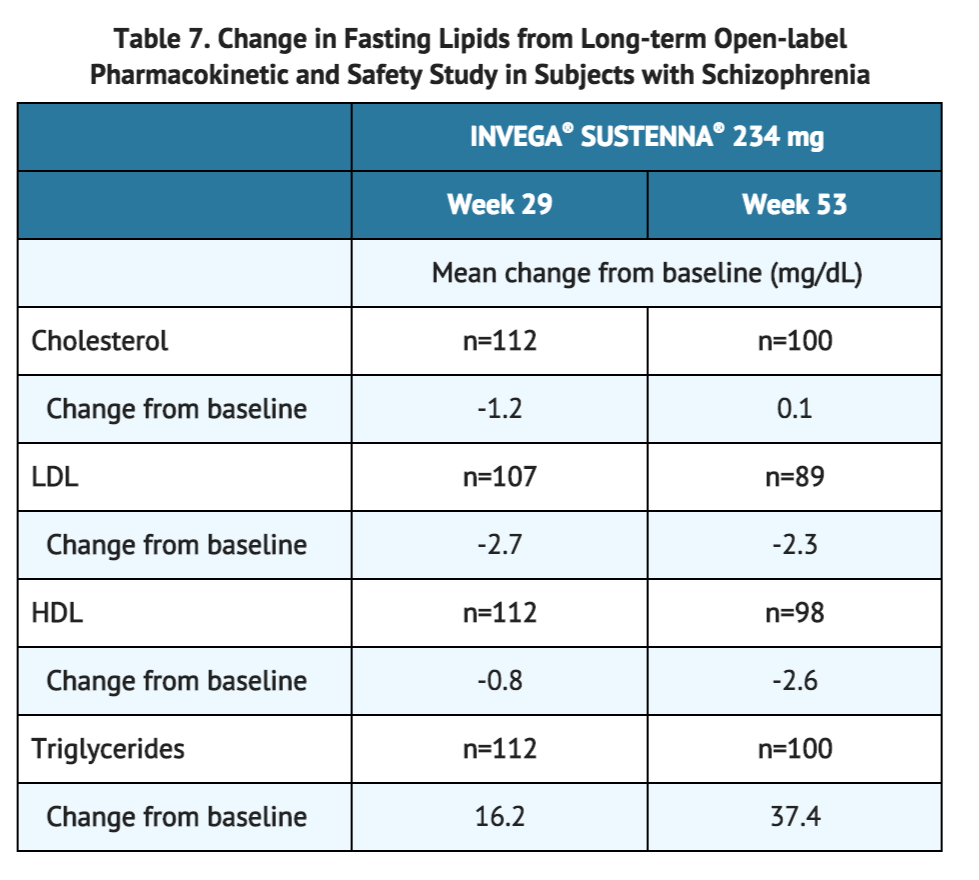
In a long-term open-label pharmacokinetic and safety study in subjects with schizophrenia in which the highest dose available (234 mg) was evaluated, INVEGA® SUSTENNA® was associated with a mean change in glucose of -0.4 mg/dL at Week 29 (n=109) and +6.8 mg/dL at Week 53 (n=100).
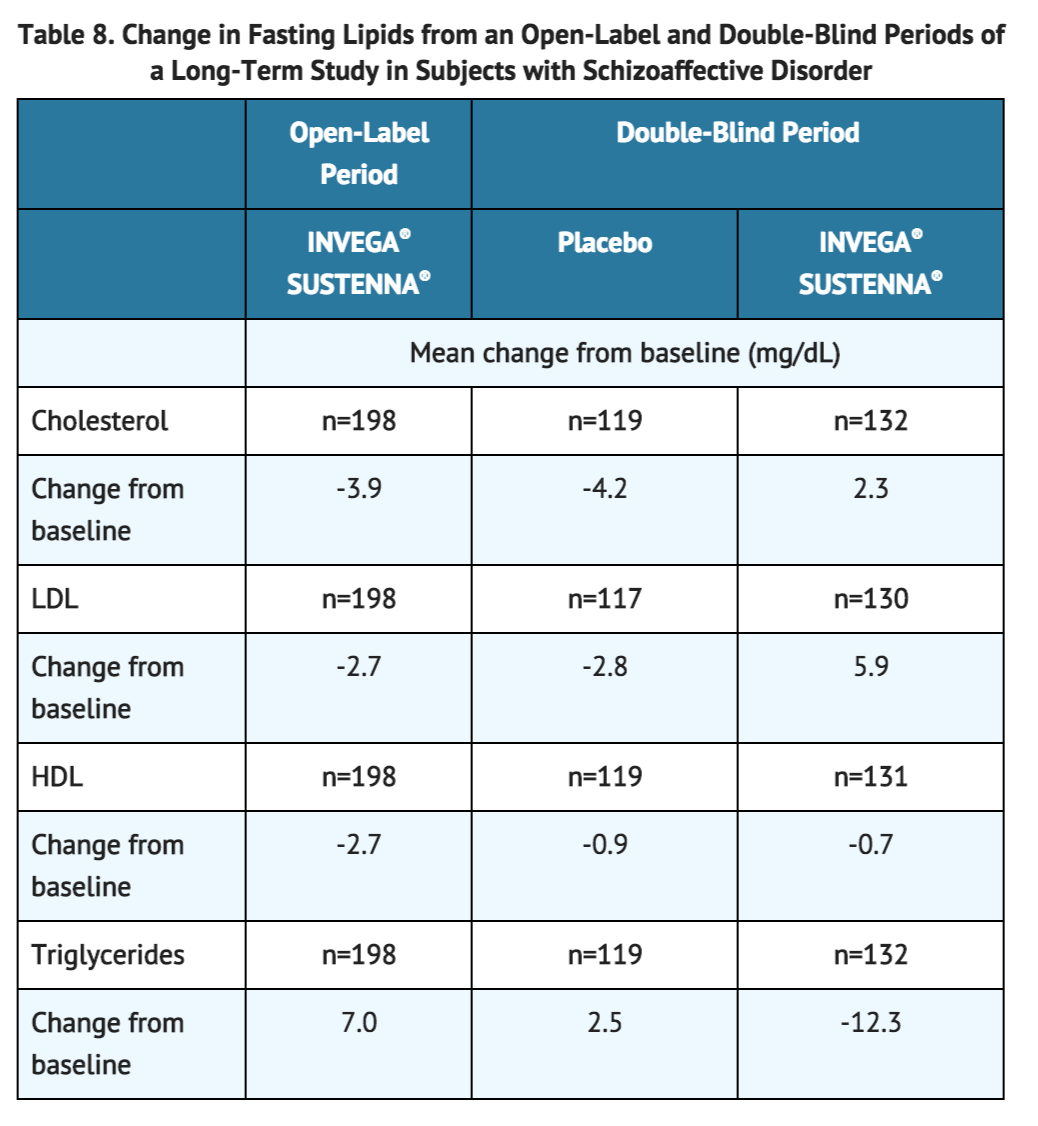
During the initial 25-week open-label period of a long-term study in subjects with schizoaffective disorder, INVEGA® SUSTENNA® was associated with mean change in glucose of +5.3 mg/dL (n=518). At the endpoint of the subsequent 15-month double-blind period of the study, INVEGA® SUSTENNA® was associated with a mean change in glucose of +0.3 mg/dL (n=131) compared with a mean change of +4.0 mg/dL in the placebo group (n=120).
Dyslipidemia
Undesirable alterations in lipids have been observed in patients treated with atypical antipsychotics.
Pooled data from the four placebo-controlled (one 9-week and three 13-week), fixed-dose studies in subjects with schizophrenia are presented in Table 6.
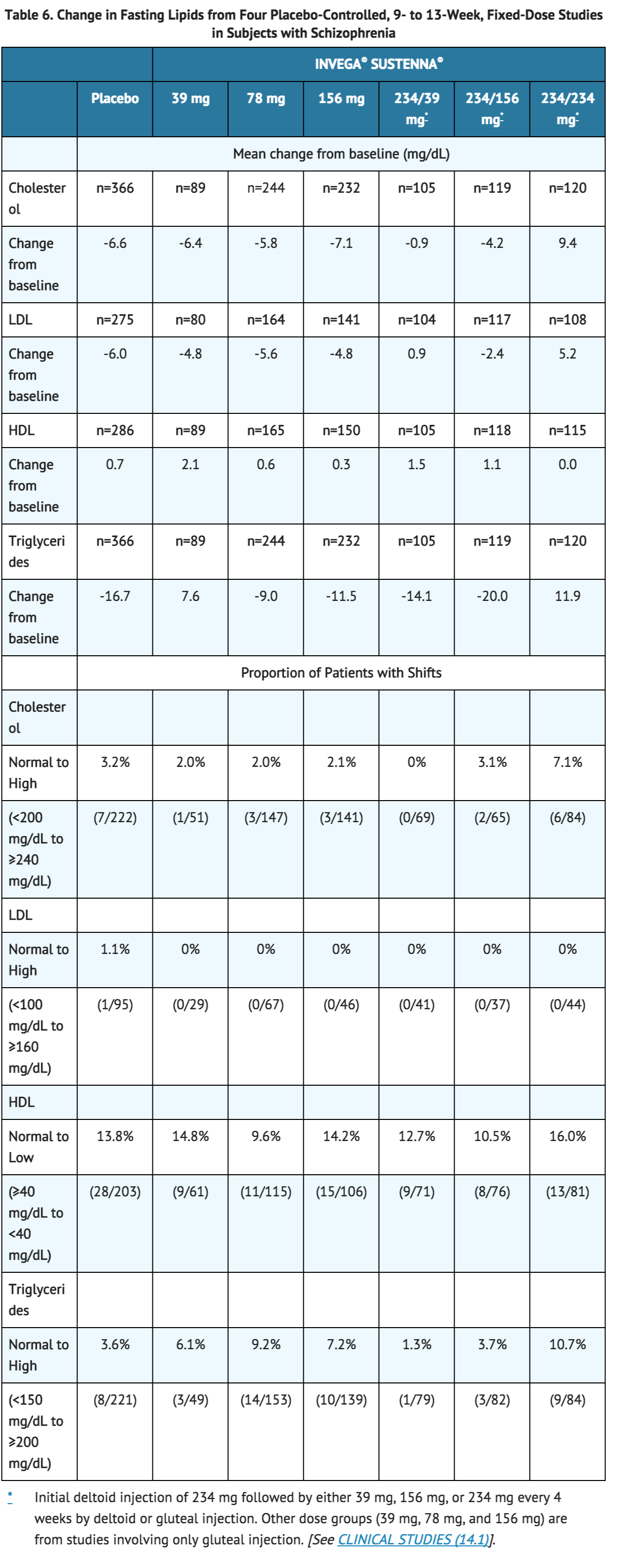
In a long-term open-label pharmacokinetic and safety study in subjects with schizophrenia in which the highest dose available (234 mg) was evaluated, the mean changes from baseline in lipid values are presented in Table 7.
The mean changes from baseline in lipid values during the initial 25-week open-label period and at the endpoint of the subsequent 15-month double-blind period in a long-term study in subjects with schizoaffective disorder are presented in Table 8.
Weight Gain
Weight gain has been observed with atypical antipsychotic use. Clinical monitoring of weight is recommended.
Data on mean changes in body weight and the proportion of subjects meeting a weight gain criterion of ≥ 7% of body weight from the four placebo-controlled (one 9-week and three 13-week), fixed-dose studies in subjects with schizophrenia are presented in Table 9.
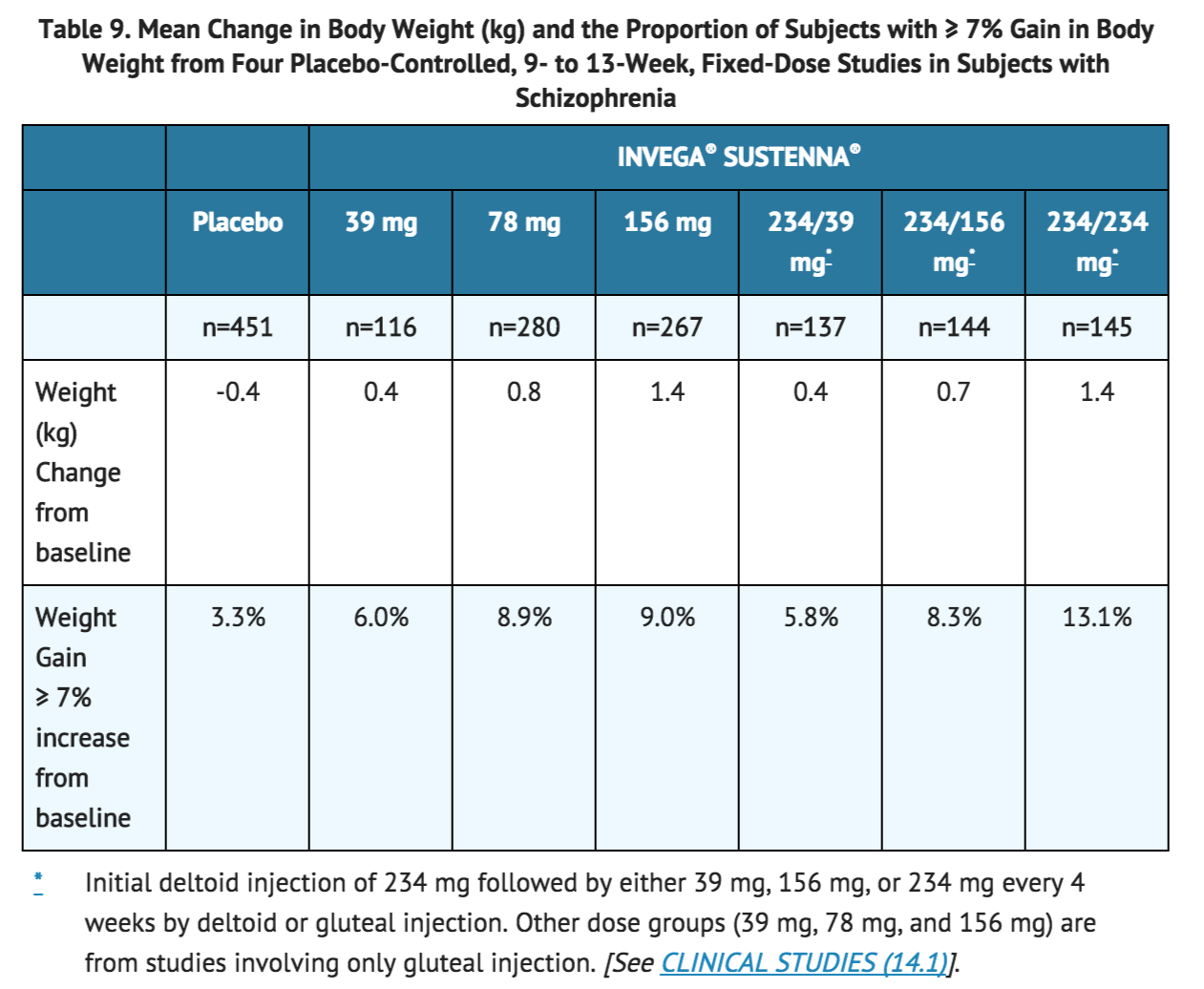
In a long-term open-label pharmacokinetic and safety study in which the highest dose available (234 mg) was evaluated, INVEGA® SUSTENNA® was associated with a mean change in weight of +2.4 kg at Week 29 (n=134) and +4.3 kg at Week 53 (n=113).
During the initial 25-week open-label period of a long-term study in subjects with schizoaffective disorder, INVEGA® SUSTENNA® was associated with a mean change in weight of +2.2 kg and 18.4% of subjects had an increase in body weight of ≥ 7% (n=653). At the endpoint of the subsequent 15-month double-blind period of the study, INVEGA® SUSTENNA® was associated with a mean change in weight of -0.2 kg and 13.0% of subjects had an increase in body weight of ≥ 7% (n=161); the placebo group had a mean change in weight of -0.8 kg and 6.0% of subjects had an increase in body weight of ≥ 7% (n=168).
5.7 Orthostatic Hypotension and Syncope Paliperidone can induce orthostatic hypotension and syncope in some patients because of its alpha-blocking activity. Syncope was reported in < 1% (4/1293) of subjects treated with INVEGA® SUSTENNA® in the recommended dose range of 39 mg to 234 mg in the four fixed-dose, double-blind, placebo-controlled trials compared with 0% (0/510) of subjects treated with placebo. In the four fixed-dose efficacy studies in subjects with schizophrenia, orthostatic hypotension was reported as an adverse event by < 1% (2/1293) of INVEGA® SUSTENNA®-treated subjects compared to 0% (0/510) with placebo. Incidences of orthostatic hypotension and syncope in the long-term studies in subjects with schizophrenia and schizoaffective disorder were similar to those observed in the short-term studies.
INVEGA® SUSTENNA® should be used with caution in patients with known cardiovascular disease (e.g., heart failure, history of myocardial infarction or ischemia, conduction abnormalities), cerebrovascular disease, or conditions that predispose the patient to hypotension (e.g., dehydration, hypovolemia, and treatment with antihypertensive medications). Monitoring of orthostatic vital signs should be considered in patients who are vulnerable to hypotension.
5.8 Leukopenia, Neutropenia, and Agranulocytosis In clinical trial and/or postmarketing experience, events of leukopenia/neutropenia have been reported temporally related to antipsychotic agents, including INVEGA®, an oral form of paliperidone. Agranulocytosis has also been reported.
Possible risk factors for leukopenia/neutropenia include pre-existing low white blood cell count (WBC) and history of drug-induced leukopenia/neutropenia. Patients with a history of a clinically significant low WBC or a drug-induced leukopenia/neutropenia should have their complete blood count (CBC) monitored frequently during the first few months of therapy and discontinuation of INVEGA® SUSTENNA® should be considered at the first sign of a clinically significant decline in WBC in the absence of other causative factors.
Patients with clinically significant neutropenia should be carefully monitored for fever or other symptoms or signs of infection and treated promptly if such symptoms or signs occur. Patients with severe neutropenia (absolute neutrophil count <1000/mm3) should discontinue INVEGA® SUSTENNA® and have their WBC followed until recovery.
5.9 Hyperprolactinemia Like other drugs that antagonize dopamine D2 receptors, paliperidone elevates prolactin levels and the elevation persists during chronic administration. Paliperidone has a prolactin-elevating effect similar to that seen with risperidone, a drug that is associated with higher levels of prolactin than other antipsychotic drugs.
Hyperprolactinemia, regardless of etiology, may suppress hypothalamic GnRH, resulting in reduced pituitary gonadotrophin secretion. This, in turn, may inhibit reproductive function by impairing gonadal steroidogenesis in both female and male patients. Galactorrhea, amenorrhea, gynecomastia, and impotence have been reported in patients receiving prolactin-elevating compounds. Long-standing hyperprolactinemia when associated with hypogonadism may lead to decreased bone density in both female and male subjects.
Tissue culture experiments indicate that approximately one-third of human breast cancers are prolactin dependent in vitro, a factor of potential importance if the prescription of these drugs is considered in a patient with previously detected breast cancer. An increase in the incidence of pituitary gland, mammary gland, and pancreatic islet cell neoplasia (mammary adenocarcinomas, pituitary and pancreatic adenomas) was observed in the risperidone carcinogenicity studies conducted in mice and rats [see NONCLINICAL TOXICOLOGY (13.1)]. Neither clinical studies nor epidemiologic studies conducted to date have shown an association between chronic administration of this class of drugs and tumorigenesis in humans, but the available evidence is too limited to be conclusive.
5.10 Potential for Cognitive and Motor Impairment Somnolence, sedation, and dizziness were reported as adverse reactions in subjects treated with INVEGA® SUSTENNA®[see ADVERSE REACTIONS (6.1)]. Antipsychotics, including INVEGA® SUSTENNA®, have the potential to impair judgment, thinking, or motor skills. Patients should be cautioned about performing activities requiring mental alertness, such as operating hazardous machinery or operating a motor vehicle, until they are reasonably certain that paliperidone therapy does not adversely affect them.
5.11 Seizures In the four fixed-dose double-blind placebo-controlled studies in subjects with schizophrenia, <1% (1/1293) of subjects treated with INVEGA® SUSTENNA® in the recommended dose range of 39 mg to 234 mg experienced an adverse event of convulsion compared with <1% (1/510) of placebo-treated subjects who experienced an adverse event of grand mal convulsion.
Like other antipsychotic drugs, INVEGA® SUSTENNA® should be used cautiously in patients with a history of seizures or other conditions that potentially lower the seizure threshold. Conditions that lower the seizure threshold may be more prevalent in patients 65 years or older.
5.12 Dysphagia Esophageal dysmotility and aspiration have been associated with antipsychotic drug use. Aspiration pneumonia is a common cause of morbidity and mortality in patients with advanced Alzheimer's dementia. INVEGA® SUSTENNA® and other antipsychotic drugs should be used cautiously in patients at risk for aspiration pneumonia.
5.13 Priapism Drugs with alpha-adrenergic blocking effects have been reported to induce priapism. Although no cases of priapism have been reported in clinical trials with INVEGA® SUSTENNA®, priapism has been reported with oral paliperidone during postmarketing surveillance. Severe priapism may require surgical intervention.
5.14 Disruption of Body Temperature Regulation Disruption of the body's ability to reduce core body temperature has been attributed to antipsychotic agents. Appropriate care is advised when prescribing INVEGA® SUSTENNA® to patients who will be experiencing conditions which may contribute to an elevation in core body temperature, e.g., exercising strenuously, exposure to extreme heat, receiving concomitant medication with anticholinergic activity, or being subject to dehydration.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Commonly Reported Adverse Reactions in Double-Blind, Placebo-Controlled Clinical Trials
Table 10 lists the adverse reactions reported in 2% or more of INVEGA® SUSTENNA®-treated subjects and at a greater proportion than in the placebo group with schizophrenia in the four fixed-dose, double-blind, placebo-controlled trials.
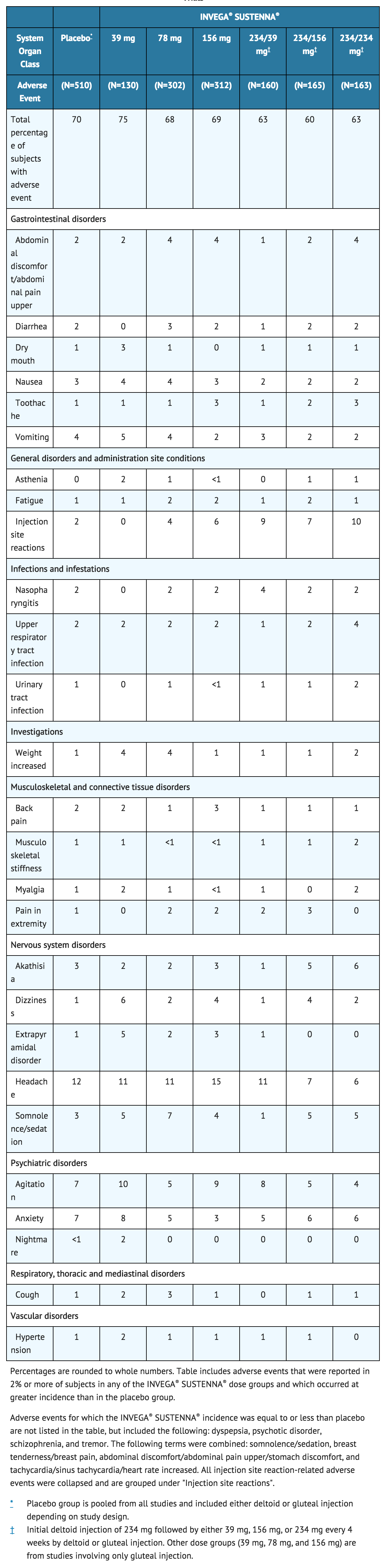
Other Adverse Reactions Observed During the Clinical Trial Evaluation of INVEGA® SUSTENNA®
The following list does not include reactions: 1) already listed in previous tables or elsewhere in labeling, 2) for which a drug cause was remote, 3) which were so general as to be uninformative, or 4) which were not considered to have significant clinical implications.
Cardiac disorders: atrioventricular block first degree, bradycardia, bundle branch block, palpitations, postural orthostatic tachycardia syndrome, tachycardia
Ear and labyrinth disorders: vertigo
Eye disorders: eye movement disorder, eye rolling, oculogyric crisis, vision blurred
Gastrointestinal disorders: constipation, dyspepsia, flatulence, salivary hypersecretion
Immune system disorders: hypersensitivity
Investigations: alanine aminotransferase increased, aspartate aminotransferase increased, electrocardiogram abnormal
Metabolism and nutrition disorders: decreased appetite, hyperinsulinemia, increased appetite
Musculoskeletal and connective tissue disorders: arthralgia, joint stiffness, muscle rigidity, muscle spasms, muscle tightness, muscle twitching, nuchal rigidity
Nervous system disorders: bradykinesia, cerebrovascular accident, convulsion, dizziness postural, drooling, dysarthria, dyskinesia, dystonia, hypertonia, lethargy, oromandibular dystonia, parkinsonism, psychomotor hyperactivity, syncope
Psychiatric disorders: insomnia, restlessness
Reproductive system and breast disorders: amenorrhea, breast discharge, erectile dysfunction, galactorrhea, gynecomastia, menstrual disorder, menstruation delayed, menstruation irregular, sexual dysfunction
Respiratory, thoracic and mediastinal disorders: nasal congestion
Skin and subcutaneous tissue disorders: drug eruption, pruritus, pruritus generalized, rash, urticaria
Discontinuations Due to Adverse Events
The percentage of subjects who discontinued due to adverse events in the four fixed-dose, double-blind, placebo-controlled schizophrenia trials were similar for INVEGA® SUSTENNA®- and placebo-treated subjects.
The percentage of subjects who discontinued due to adverse events in the open-label period of the long-term study in subjects with schizoaffective disorder was 7.5%. During the double-blind, placebo-controlled period of that study, the percentages of subjects who discontinued due to adverse events were 5.5% and 1.8% in INVEGA® SUSTENNA®- and placebo-treated subjects, respectively.
Dose-Related Adverse Reactions
Based on the pooled data from the four fixed-dose, double-blind, placebo-controlled trials in subjects with schizophrenia, among the adverse reactions that occurred at ≥ 2% incidence in the subjects treated with INVEGA® SUSTENNA®, only akathisia increased with dose. Hyperprolactinemia also exhibited a dose relationship, but did not occur at ≥ 2% incidence in INVEGA® SUSTENNA®-treated subjects from the four fixed-dose studies.
Demographic Differences
An examination of population subgroups in the double-blind placebo-controlled trials did not reveal any evidence of differences in safety on the basis of age, gender, or race alone; however, there were few subjects ≥ 65 years of age.
Extrapyramidal Symptoms (EPS)
Pooled data from the two double-blind, placebo-controlled, 13-week, fixed-dose trials in adult subjects with schizophrenia provided information regarding EPS. Several methods were used to measure EPS: (1) the Simpson-Angus global score (mean change from baseline or score at the end of trial) which broadly evaluates Parkinsonism, (2) the Barnes Akathisia Rating Scale global clinical rating score (mean change from baseline or score at the end of trial) which evaluates akathisia, (3) use of anticholinergic medications to treat EPS, (4) the Abnormal Involuntary Movement Scale scores (mean change from baseline or scores at the end of trial) (Table 11), and (5) incidence of spontaneous reports of EPS (Table 12).
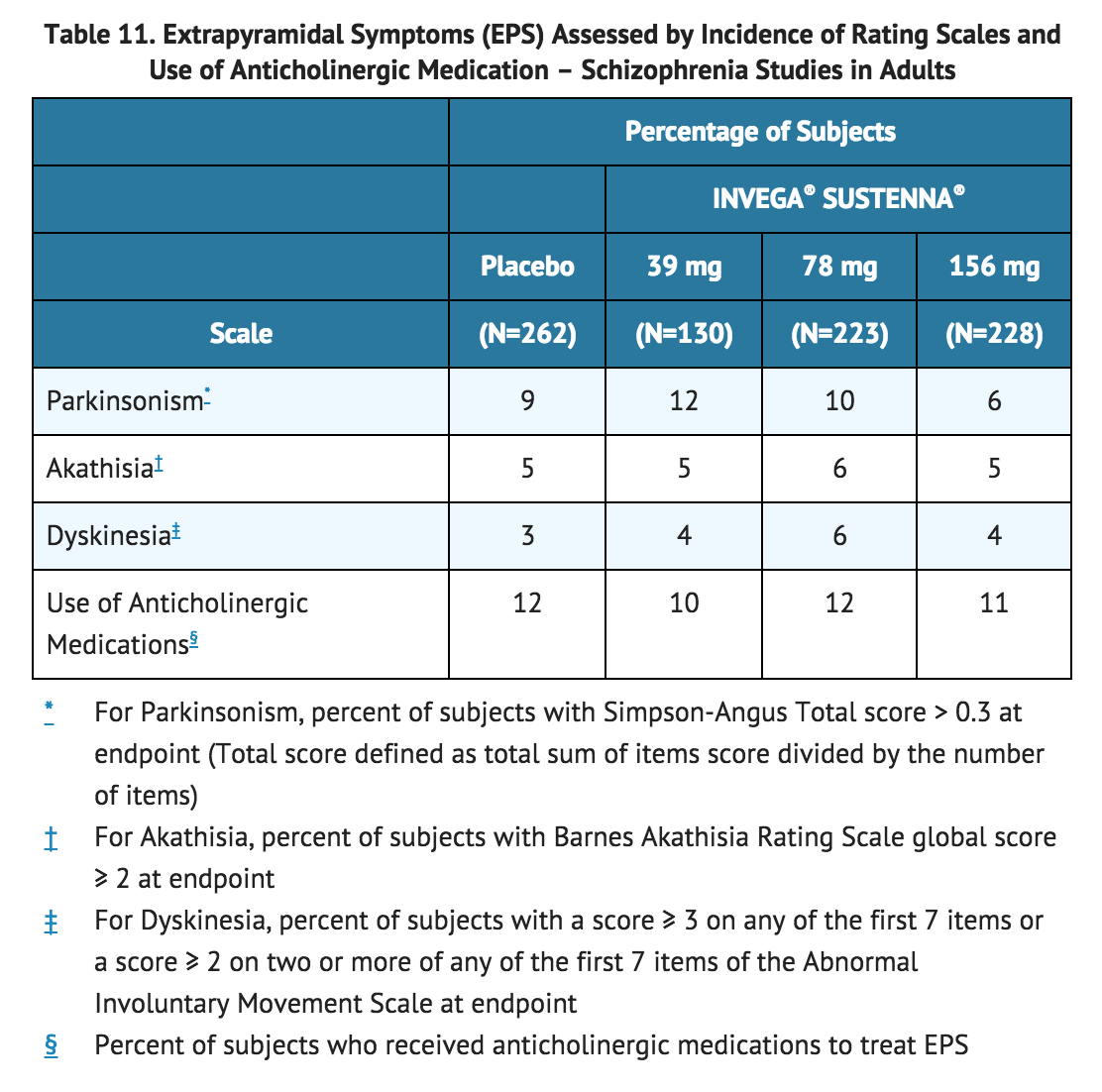
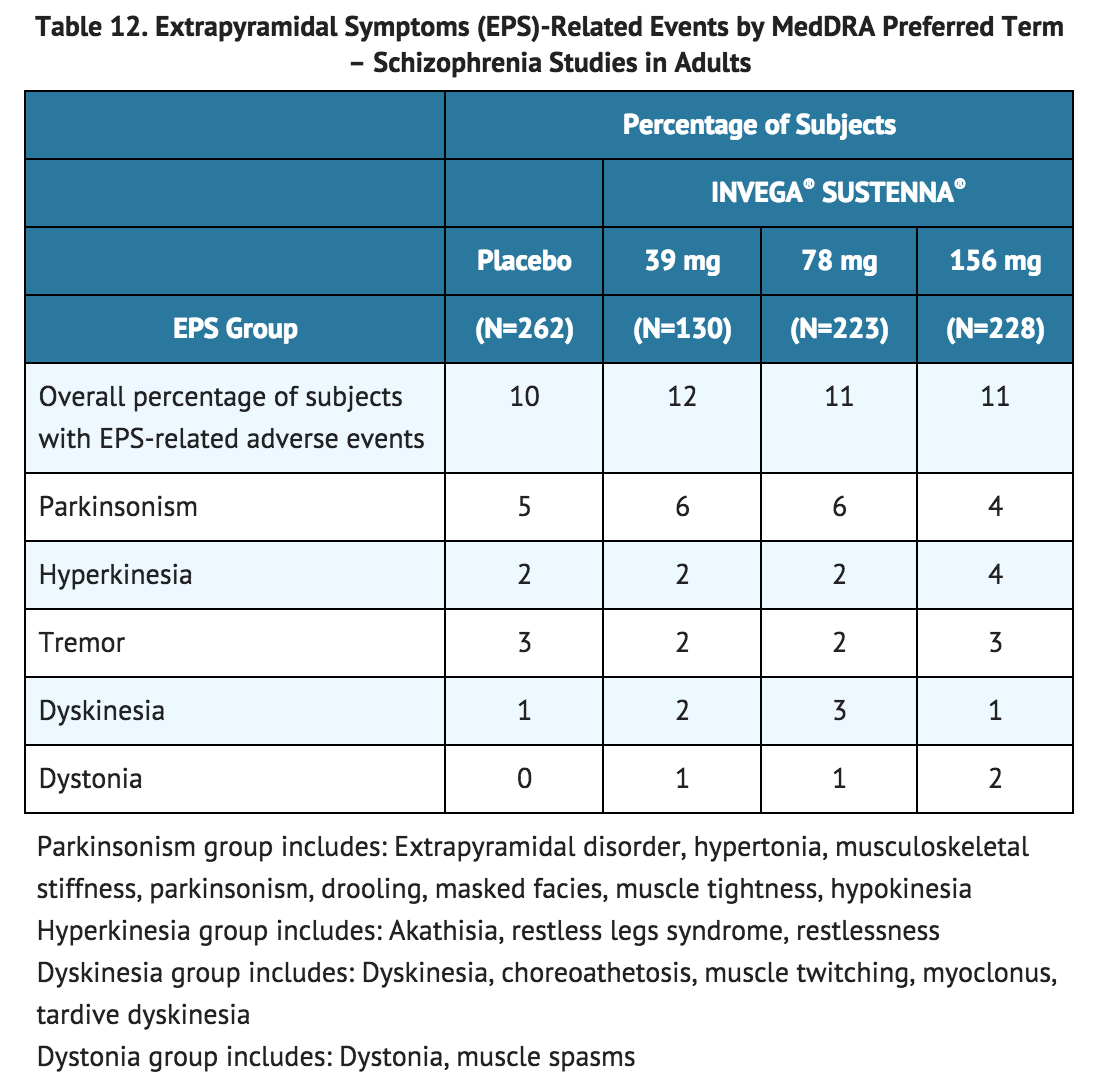
The results across all phases of the maintenance trial in subjects with schizophrenia exhibited comparable findings. In the 9-week, fixed-dose, double-blind, placebo-controlled trial, the proportions of Parkinsonism and akathisia assessed by incidence of rating scales were higher in the INVEGA® SUSTENNA® 156 mg group (18% and 11%, respectively) than in the INVEGA® SUSTENNA® 78 mg group (9% and 5%, respectively) and placebo group (7% and 4%, respectively).
In the 13-week study in subjects with schizophrenia involving 234 mg initiation dosing, the incidence of any EPS was similar to that of the placebo group (8%), but exhibited a dose-related pattern with 6%, 10%, and 11% in the INVEGA® SUSTENNA® 234/39 mg, 234/156 mg, and 234/234 mg groups, respectively. Hyperkinesia was the most frequent category of EPS-related adverse events in this study, and was reported at a similar rate between the placebo (4.9%) and INVEGA® SUSTENNA® 234/156 mg (4.8%) and 234/234 mg (5.5%) groups, but at a lower rate in the 234/39 mg group (1.3%).
In the long-term study in subjects with schizoaffective disorder, the EPS during the 25-week open-label INVEGA® SUSTENNA® treatment were hyperkinesia (12.3%), parkinsonism (8.7%), tremor (3.4%), dyskinesia (2.5%), and dystonia (2.1%). During the 15-month double-blind treatment, the incidence of any EPS was similar to that of the placebo group (8.5% and 7.1% respectively). The most commonly reported treatment-emergent EPS-related adverse events (>2%) in any treatment group in the double-blind phase of the study (INVEGA® SUSTENNA® versus placebo) were hyperkinesia (3.7% vs. 2.9%), parkinsonism (3.0% vs. 1.8%), and tremor (1.2% vs. 2.4%).
Dystonia
Symptoms of dystonia, prolonged abnormal contractions of muscle groups, may occur in susceptible individuals during the first few days of treatment. Dystonic symptoms include: spasm of the neck muscles, sometimes progressing to tightness of the throat, swallowing difficulty, difficulty breathing, and/or protrusion of the tongue. While these symptoms can occur at low doses, they occur more frequently and with greater severity with high potency and at higher doses of first generation antipsychotic drugs. An elevated risk of acute dystonia is observed in males and younger age groups.
Laboratory Test Abnormalities
In the pooled data from the two double-blind, placebo-controlled, 13-week, fixed-dose trials in subjects with schizophrenia, a between-group comparison revealed no medically important differences between INVEGA® SUSTENNA® and placebo in the proportions of subjects experiencing potentially clinically significant changes in routine serum chemistry, hematology, or urinalysis parameters. Similarly, there were no differences between INVEGA® SUSTENNA® and placebo in the incidence of discontinuations due to changes in hematology, urinalysis, or serum chemistry, including mean changes from baseline in fasting glucose, insulin, c-peptide, triglycerides, HDL, LDL, and total cholesterol measurements. However, INVEGA® SUSTENNA® was associated with increases in serum prolactin [see WARNINGS AND PRECAUTIONS (5.9)]. The results from the 13-week study involving 234 mg initiation dosing, the 9-week, fixed-dose, double-blind, placebo-controlled trial, and the double-blind phase of the maintenance trial in subjects with schizophrenia exhibited comparable findings.
Pain Assessment and Local Injection Site Reactions
In the pooled data from the two 13-week, fixed-dose, double-blind, placebo-controlled trials in subjects with schizophrenia, the mean intensity of injection pain reported by subjects using a visual analog scale (0 = no pain to 100 = unbearably painful) decreased in all treatment groups from the first to the last injection (placebo: 10.9 to 9.8; 39 mg: 10.3 to 7.7; 78 mg: 10.0 to 9.2; 156 mg: 11.1 to 8.8). The results from both the 9-week, fixed-dose, double-blind, placebo-controlled trial and the double-blind phase of the maintenance trial exhibited comparable findings.
In the 13-week study involving 234 mg initiation dosing in subjects with schizophrenia, occurrences of induration, redness, or swelling, as assessed by blinded study personnel, were infrequent, generally mild, decreased over time, and similar in incidence between the INVEGA® SUSTENNA® and placebo groups. Investigator ratings of injection pain were similar for the placebo and INVEGA® SUSTENNA® groups. Investigator evaluations of the injection site after the first injection for redness, swelling, induration, and pain were rated as absent for 69–100% of subjects in both the INVEGA® SUSTENNA® and placebo groups. At Day 92, investigators rated absence of redness, swelling, induration, and pain in 95–100% of subjects in both the INVEGA® SUSTENNA® and placebo groups.
Adverse Reactions Reported in Clinical Trials with Oral Paliperidone
The following is a list of additional adverse reactions that have been reported in clinical trials with oral paliperidone:
Cardiac disorders: bundle branch block left, sinus arrhythmia
Gastrointestinal disorders: abdominal pain, small intestinal obstruction
General disorders and administration site conditions: edema, edema peripheral
Immune system disorders: anaphylactic reaction
Infections and infestations: rhinitis
Musculoskeletal and connective tissue disorders: musculoskeletal pain, torticollis, trismus
Nervous system disorders: cogwheel rigidity, grand mal convulsion, parkinsonian gait, transient ischemic attack
Psychiatric disorders: sleep disorder
Reproductive system and breast disorders: breast engorgement, breast tenderness/breast pain, retrograde ejaculation
Respiratory, thoracic and mediastinal disorders: pharyngolaryngeal pain, pneumonia aspiration
Skin and subcutaneous tissue disorders: rash papular
Vascular disorders: hypotension, ischemia
6.2 Postmarketing Experience The following adverse reactions have been identified during postapproval use of paliperidone; because these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Reactions already listed in other parts of ADVERSE REACTIONS (6), or those considered in WARNINGS AND PRECAUTIONS (5) are not listed here.
Blood disorders: thrombotic thrombocytopenic purpura
Gastrointestinal disorders: ileus
Genitourinary disorders: urinary incontinence, urinary retention
Immune system disorders: angioedema, swollen tongue
Cases of anaphylactic reaction after injection with INVEGA® SUSTENNA® have been reported during postmarketing experience in patients who have previously tolerated oral risperidone or oral paliperidone.
6.3 Adverse Reactions Reported With Risperidone Paliperidone is the major active metabolite of risperidone. Adverse reactions reported with oral risperidone and risperidone long-acting injection can be found in the ADVERSE REACTIONS sections of the package inserts for those products.
Postmarketing Experience
There is limited information regarding Paliperidone (injection) Postmarketing Experience in the drug label.
Drug Interactions
Because paliperidone palmitate is hydrolyzed to paliperidone [see CLINICAL PHARMACOLOGY (12.3)], results from studies with oral paliperidone should be taken into consideration when assessing drug-drug interaction potential.
7.1 Potential for INVEGA® SUSTENNA® to Affect Other Drugs Paliperidone may antagonize the effect of levodopa and other dopamine agonists.
Because of its potential for inducing orthostatic hypotension, an additive effect may occur when INVEGA® SUSTENNA® is administered with other therapeutic agents that have this potential [see WARNINGS AND PRECAUTIONS (5.7)].
No dose adjustment is necessary for lithium when it is coadministered with INVEGA® SUSTENNA®. Pharmacokinetic interaction between INVEGA® SUSTENNA® and lithium is unlikely.
No dose adjustment is necessary for valproate when INVEGA® SUSTENNA® is added to the therapy. Steady-state pharmacokinetics of valproate was not affected when patients were coadministered oral paliperidone extended-release tablets [see CLINICAL PHARMACOLOGY (12.3)].
Paliperidone is not expected to cause clinically important pharmacokinetic interactions with drugs that are metabolized by cytochrome P450 isozymes [see CLINICAL PHARMACOLOGY (12.3)].
7.2 Potential for Other Drugs to Affect INVEGA® SUSTENNA® On initiation of strong inducers of both CYP3A4 and P-gp (e.g., carbamazepine, rifampin, or St John's wort), it may be necessary to increase the dose of INVEGA® SUSTENNA®. Conversely, on discontinuation of the strong inducer, it may be necessary to decrease the dose of INVEGA® SUSTENNA® [see CLINICAL PHARMACOLOGY (12.3)].
No dose adjustment is necessary for INVEGA® SUSTENNA® when valproate is added to treatment [see CLINICAL PHARMACOLOGY (12.3)].
No dose adjustment is necessary for INVEGA® SUSTENNA® when it is coadministered with lithium. Pharmacokinetic interaction between INVEGA® SUSTENNA® and lithium is unlikely.
In vitro studies indicate that CYP2D6 and CYP3A4 may be involved in paliperidone metabolism; however, there is no evidence in vivo that inhibitors of these enzymes significantly affect the metabolism of paliperidone. Paliperidone is not a substrate of CYP1A2, CYP2A6, CYP2C9, and CYP2C19; an interaction with inhibitors or inducers of these isozymes is unlikely.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Paliperidone (injection) in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Paliperidone (injection) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Paliperidone (injection) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Paliperidone (injection) in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Paliperidone (injection) in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Paliperidone (injection) in geriatric settings.
Gender
There is no FDA guidance on the use of Paliperidone (injection) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Paliperidone (injection) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Paliperidone (injection) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Paliperidone (injection) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Paliperidone (injection) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Paliperidone (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Paliperidone (injection) Administration in the drug label.
Monitoring
There is limited information regarding Paliperidone (injection) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Paliperidone (injection) and IV administrations.
Overdosage
There is limited information regarding Paliperidone (injection) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Paliperidone (injection) Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Paliperidone (injection) Mechanism of Action in the drug label.
Structure
There is limited information regarding Paliperidone (injection) Structure in the drug label.
Pharmacodynamics
There is limited information regarding Paliperidone (injection) Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Paliperidone (injection) Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Paliperidone (injection) Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Paliperidone (injection) Clinical Studies in the drug label.
How Supplied
There is limited information regarding Paliperidone (injection) How Supplied in the drug label.
Storage
There is limited information regarding Paliperidone (injection) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Paliperidone (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Paliperidone (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Paliperidone (injection) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Paliperidone (injection) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Paliperidone (injection) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Paliperidone (injection) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.