Paliperidone (injection): Difference between revisions
(Created page with "{{DrugProjectFormSinglePage |indicationType=treatment |blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> |blackBoxWarningBody=<i><span style="color:#FF0000...") |
No edit summary |
||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag={{TA}} | |||
|aOrAn=an | |||
|drugClass= atypical antipsychotic | |||
|indicationType=treatment | |indicationType=treatment | ||
|blackBoxWarningTitle=<b><span style="color:#FF0000;"> | |indication=schizophrenia | ||
|blackBoxWarningBody= | |hasBlackBoxWarning=Yes | ||
|adverseReactions=injection site reactions, somnolence/sedation, dizziness, akathisia, and extrapyramidal disorder | |||
|blackBoxWarningTitle=<b><span style="color:#FF0000;">WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS</span></b> | |||
|blackBoxWarningBody=Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death [see WARNINGS AND PRECAUTIONS (5.1)]. | |||
INVEGA® SUSTENNA® is not approved for use in patients with dementia-related psychosis [see WARNINGS AND PRECAUTIONS (5.1)]. | |||
|fdaLIADAdult====Indications=== | |||
INVEGA® SUSTENNA® (paliperidone palmitate) is indicated for the treatment of: | |||
Schizophrenia [see CLINICAL STUDIES 14.1]. | |||
Schizoaffective disorder as monotherapy and as an adjunct to mood stabilizers or antidepressants [see CLINICAL STUDIES 14.2]. | |||
===Dosage=== | |||
2.1 Administration Instructions | |||
Each injection must be administered only by a health care professional. | |||
Parenteral drug products should be inspected visually for foreign matter and discoloration prior to administration, whenever product and container permit. | |||
INVEGA® SUSTENNA® is intended for intramuscular use only. Do not administer by any other route. Avoid inadvertent injection into a blood vessel. Administer the dose in a single injection; do not administer the dose in divided injections. Inject slowly, deep into the muscle. | |||
The recommended needle size for administration of INVEGA® SUSTENNA® into the deltoid muscle is determined by the patient's weight: | |||
For patients weighing less than 90 kg, the 1-inch, 23 gauge needle is recommended. | |||
For patients weighing 90 kg or more, the 1½-inch, 22 gauge needle is recommended. | |||
Deltoid injections should be alternated between the two deltoid muscles. | |||
The recommended needle size for administration of INVEGA® SUSTENNA® into the gluteal muscle is the 1½-inch, 22 gauge needle regardless of patient weight. | |||
Administer into the upper-outer quadrant of the gluteal muscle. Gluteal injections should be alternated between the two gluteal muscles. | |||
2.2 Schizophrenia and Schizoaffective Disorder | |||
For patients who have never taken oral paliperidone or oral or injectable risperidone, it is recommended to establish tolerability with oral paliperidone or oral risperidone prior to initiating treatment with INVEGA® SUSTENNA®. | |||
The recommended dosing of INVEGA® SUSTENNA® for each approved indication is displayed in Table 1. The recommended initiation of INVEGA® SUSTENNA® is with a dose of 234 mg on treatment day 1 and 156 mg one week later, both administered in the deltoid muscle. Following the second initiation dose, monthly maintenance doses can be administered in either the deltoid or gluteal muscle. | |||
[[File:INVEGA1.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
Adjustment of the maintenance dose may be made monthly. When making dose adjustments, the prolonged-release characteristics of INVEGA® SUSTENNA® should be considered [see CLINICAL PHARMACOLOGY (12.3)], as the full effect of the dose adjustment may not be evident for several months. | |||
2.3 Missed Doses | |||
Avoiding Missed Doses | |||
It is recommended that the second initiation dose of INVEGA® SUSTENNA® be given one week after the first dose. To avoid a missed dose, patients may be given the second dose 4 days before or after the one-week time point. Similarly, the third and subsequent injections after the initiation regimen are recommended to be given monthly. To avoid a missed monthly dose, patients may be given the injection up to 7 days before or after the monthly time point. | |||
Management of a Missed Second Initiation Dose | |||
If the target date for the second INVEGA® SUSTENNA® injection (one week ± 4 days) is missed, the recommended reinitiation depends on the length of time which has elapsed since the patient's first injection. In case of a missed second initiation dose follow the dosing instructions provided in Table 2. | |||
[[File:INVEGA2.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
2.4 Use with Oral Paliperidone or with Risperidone | |||
Concomitant use of INVEGA® SUSTENNA® with oral paliperidone or oral or injectable risperidone has not been studied. Since paliperidone is the major active metabolite of risperidone, consideration should be given to the additive paliperidone exposure if any of these medications are coadministered with INVEGA® SUSTENNA®. | |||
2.5 Dosage Adjustments | |||
Renal Impairment | |||
INVEGA® SUSTENNA® has not been systematically studied in patients with renal impairment [see CLINICAL PHARMACOLOGY (12.3)]. For patients with mild renal impairment (creatinine clearance ≥ 50 mL/min to < 80 mL/min [Cockcroft-Gault Formula]), initiate INVEGA® SUSTENNA® with a dose of 156 mg on treatment day 1 and 117 mg one week later. Administer both doses in the deltoid muscle. Thereafter, follow with monthly injections of 78 mg in either the deltoid or gluteal muscle [see USE IN SPECIFIC POPULATIONS (8.6) and CLINICAL PHARMACOLOGY (12.3)]. | |||
INVEGA® SUSTENNA® is not recommended in patients with moderate or severe renal impairment (creatinine clearance < 50 mL/min) [see USE IN SPECIFIC POPULATIONS (8.6) and CLINICAL PHARMACOLOGY (12.3)]. | |||
Coadministration with Strong CYP3A4/P-glycoprotein (P-gp) Inducers | |||
It may be necessary to increase the dose of INVEGA® SUSTENNA® when a strong inducer of both CYP3A4 and P-gp (e.g., carbamazepine, rifampin, St John's wort) is co-administered. Conversely, on discontinuation of the strong inducer, it may be necessary to decrease the dose of INVEGA® SUSTENNA® [see DRUG INTERACTIONS (7.2) and CLINICAL PHARMACOLOGY (12.3)]. | |||
2.6 Switching from Other Antipsychotics | |||
There are no systematically collected data to specifically address switching patients with schizophrenia or schizoaffective disorder from other antipsychotics to INVEGA® SUSTENNA®, or concerning concomitant administration with other antipsychotics. | |||
2.6.1 Switching from Oral Antipsychotics | |||
For patients who have never taken oral paliperidone or oral or injectable risperidone, tolerability should be established with oral paliperidone or oral risperidone prior to initiating treatment with INVEGA® SUSTENNA®. | |||
Previous oral antipsychotics can be discontinued at the time of initiation of treatment with INVEGA® SUSTENNA®. Recommended initiation of INVEGA® SUSTENNA® is with a dose of 234 mg on treatment day 1 and 156 mg one week later, both administered in the deltoid muscle [see DOSAGE AND ADMINISTRATION (2.2)]. Patients previously stabilized on different doses of INVEGA® Extended-Release tablets can attain similar paliperidone steady-state exposure during maintenance treatment with INVEGA® SUSTENNA® monthly doses as depicted in Table 4. | |||
[[File:INVEGA3.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
2.6.2 Switching from Long-Acting Injectable Antipsychotics | |||
For patients who have never taken oral paliperidone or oral or injectable risperidone, tolerability should be established with oral paliperidone or oral risperidone prior to initiating treatment with INVEGA® SUSTENNA®. | |||
When switching patients currently at steady-state on a long-acting injectable antipsychotic, initiate INVEGA® SUSTENNA® therapy in place of the next scheduled injection. INVEGA® SUSTENNA® should then be continued at monthly intervals. The one-week initiation dosing regimen as described in Section 2.2 is not required. See TABLE 1 above for recommended monthly maintenance dosing. Based on previous clinical history of tolerability and/or efficacy, some patients may benefit from lower or higher maintenance doses within the available strengths (39 mg, 78 mg, 117 mg, 156 mg, and 234 mg). The 39 mg strength was not studied in the long-term schizoaffective disorder study. Monthly maintenance doses can be administered in either the deltoid or gluteal muscle [see DOSAGE AND ADMINISTRATION (2.2)]. | |||
If INVEGA® SUSTENNA® is discontinued, its prolonged-release characteristics must be considered. As recommended with other antipsychotic medications, the need for continuing existing extrapyramidal symptoms (EPS) medication should be re-evaluated periodically. | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Paliperidone (injection) in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Paliperidone (injection) in adult patients. | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Paliperidone (injection) in adult patients. | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Paliperidone (injection) in adult patients. | ||
Revision as of 19:22, 19 May 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Turky Alkathery, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
See full prescribing information for complete Boxed Warning.
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death [see WARNINGS AND PRECAUTIONS (5.1)].
INVEGA® SUSTENNA® is not approved for use in patients with dementia-related psychosis [see WARNINGS AND PRECAUTIONS (5.1)].
|
Overview
Paliperidone (injection) is an atypical antipsychotic that is FDA approved for the treatment of schizophrenia. There is a Black Box Warning for this drug as shown here. Common adverse reactions include injection site reactions, somnolence/sedation, dizziness, akathisia, and extrapyramidal disorder.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
INVEGA® SUSTENNA® (paliperidone palmitate) is indicated for the treatment of:
Schizophrenia [see CLINICAL STUDIES 14.1]. Schizoaffective disorder as monotherapy and as an adjunct to mood stabilizers or antidepressants [see CLINICAL STUDIES 14.2].
Dosage
2.1 Administration Instructions Each injection must be administered only by a health care professional.
Parenteral drug products should be inspected visually for foreign matter and discoloration prior to administration, whenever product and container permit.
INVEGA® SUSTENNA® is intended for intramuscular use only. Do not administer by any other route. Avoid inadvertent injection into a blood vessel. Administer the dose in a single injection; do not administer the dose in divided injections. Inject slowly, deep into the muscle.
The recommended needle size for administration of INVEGA® SUSTENNA® into the deltoid muscle is determined by the patient's weight:
For patients weighing less than 90 kg, the 1-inch, 23 gauge needle is recommended. For patients weighing 90 kg or more, the 1½-inch, 22 gauge needle is recommended. Deltoid injections should be alternated between the two deltoid muscles.
The recommended needle size for administration of INVEGA® SUSTENNA® into the gluteal muscle is the 1½-inch, 22 gauge needle regardless of patient weight.
Administer into the upper-outer quadrant of the gluteal muscle. Gluteal injections should be alternated between the two gluteal muscles.
2.2 Schizophrenia and Schizoaffective Disorder For patients who have never taken oral paliperidone or oral or injectable risperidone, it is recommended to establish tolerability with oral paliperidone or oral risperidone prior to initiating treatment with INVEGA® SUSTENNA®.
The recommended dosing of INVEGA® SUSTENNA® for each approved indication is displayed in Table 1. The recommended initiation of INVEGA® SUSTENNA® is with a dose of 234 mg on treatment day 1 and 156 mg one week later, both administered in the deltoid muscle. Following the second initiation dose, monthly maintenance doses can be administered in either the deltoid or gluteal muscle.
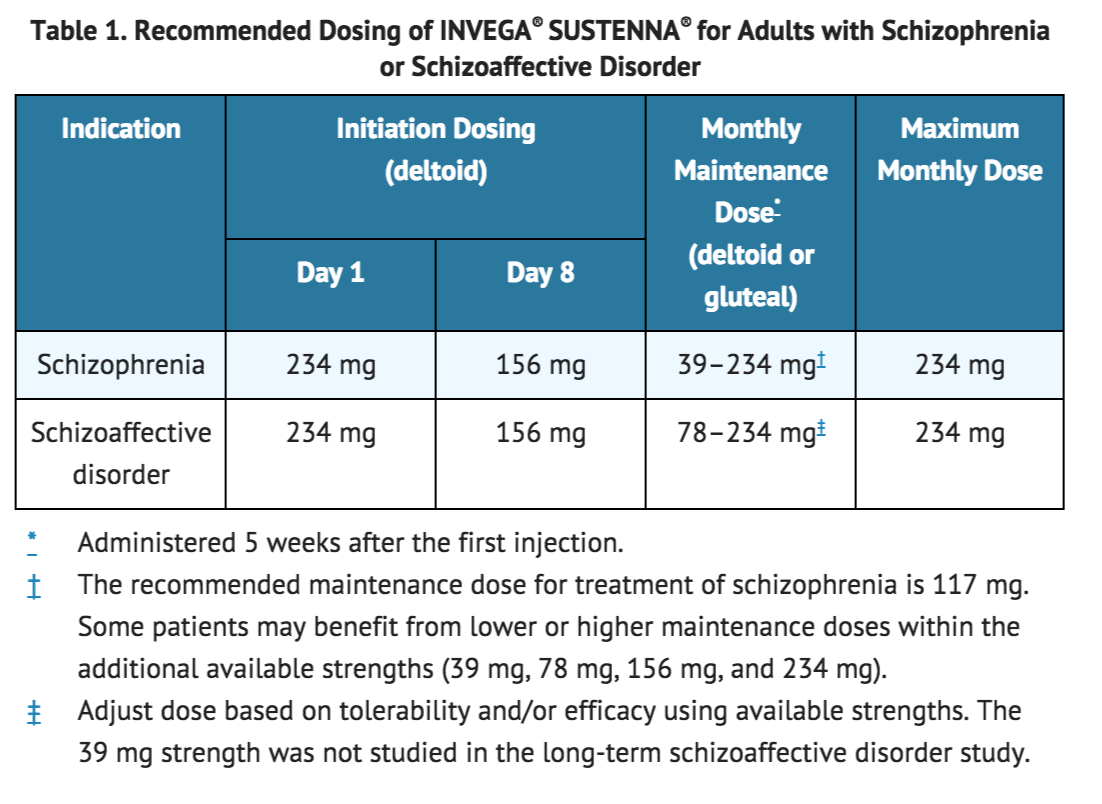
Adjustment of the maintenance dose may be made monthly. When making dose adjustments, the prolonged-release characteristics of INVEGA® SUSTENNA® should be considered [see CLINICAL PHARMACOLOGY (12.3)], as the full effect of the dose adjustment may not be evident for several months.
2.3 Missed Doses Avoiding Missed Doses
It is recommended that the second initiation dose of INVEGA® SUSTENNA® be given one week after the first dose. To avoid a missed dose, patients may be given the second dose 4 days before or after the one-week time point. Similarly, the third and subsequent injections after the initiation regimen are recommended to be given monthly. To avoid a missed monthly dose, patients may be given the injection up to 7 days before or after the monthly time point.
Management of a Missed Second Initiation Dose
If the target date for the second INVEGA® SUSTENNA® injection (one week ± 4 days) is missed, the recommended reinitiation depends on the length of time which has elapsed since the patient's first injection. In case of a missed second initiation dose follow the dosing instructions provided in Table 2.
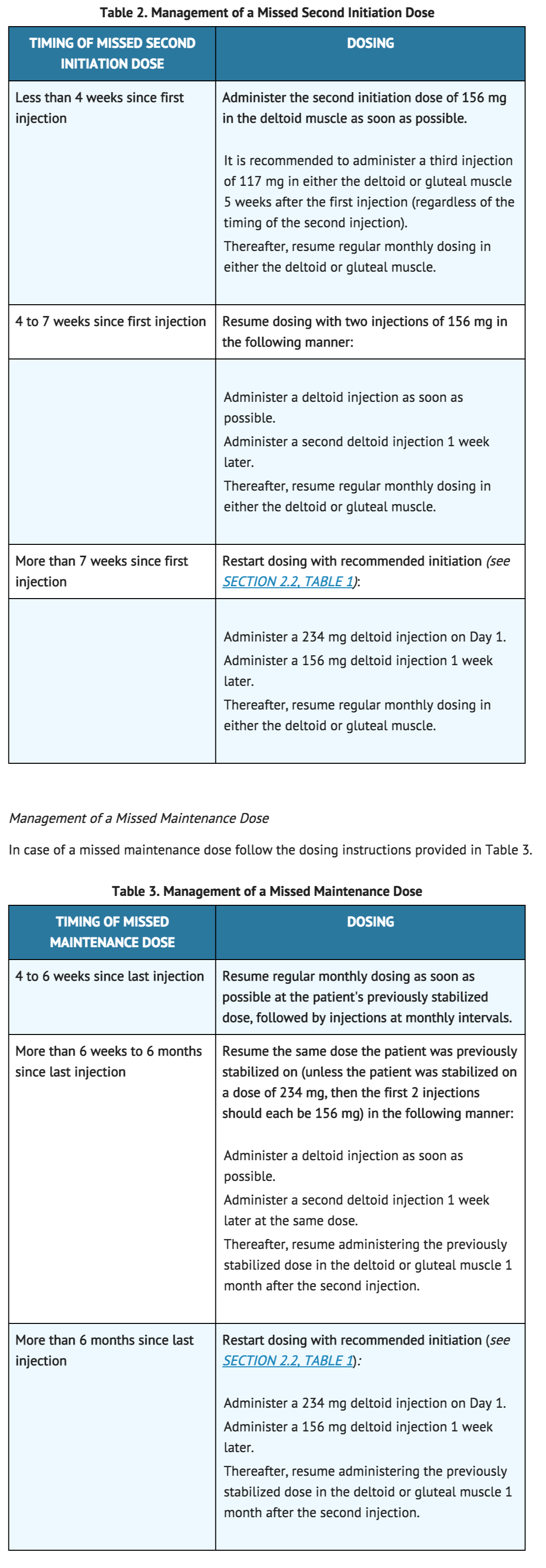
2.4 Use with Oral Paliperidone or with Risperidone Concomitant use of INVEGA® SUSTENNA® with oral paliperidone or oral or injectable risperidone has not been studied. Since paliperidone is the major active metabolite of risperidone, consideration should be given to the additive paliperidone exposure if any of these medications are coadministered with INVEGA® SUSTENNA®.
2.5 Dosage Adjustments Renal Impairment
INVEGA® SUSTENNA® has not been systematically studied in patients with renal impairment [see CLINICAL PHARMACOLOGY (12.3)]. For patients with mild renal impairment (creatinine clearance ≥ 50 mL/min to < 80 mL/min [Cockcroft-Gault Formula]), initiate INVEGA® SUSTENNA® with a dose of 156 mg on treatment day 1 and 117 mg one week later. Administer both doses in the deltoid muscle. Thereafter, follow with monthly injections of 78 mg in either the deltoid or gluteal muscle [see USE IN SPECIFIC POPULATIONS (8.6) and CLINICAL PHARMACOLOGY (12.3)].
INVEGA® SUSTENNA® is not recommended in patients with moderate or severe renal impairment (creatinine clearance < 50 mL/min) [see USE IN SPECIFIC POPULATIONS (8.6) and CLINICAL PHARMACOLOGY (12.3)].
Coadministration with Strong CYP3A4/P-glycoprotein (P-gp) Inducers
It may be necessary to increase the dose of INVEGA® SUSTENNA® when a strong inducer of both CYP3A4 and P-gp (e.g., carbamazepine, rifampin, St John's wort) is co-administered. Conversely, on discontinuation of the strong inducer, it may be necessary to decrease the dose of INVEGA® SUSTENNA® [see DRUG INTERACTIONS (7.2) and CLINICAL PHARMACOLOGY (12.3)].
2.6 Switching from Other Antipsychotics There are no systematically collected data to specifically address switching patients with schizophrenia or schizoaffective disorder from other antipsychotics to INVEGA® SUSTENNA®, or concerning concomitant administration with other antipsychotics.
2.6.1 Switching from Oral Antipsychotics
For patients who have never taken oral paliperidone or oral or injectable risperidone, tolerability should be established with oral paliperidone or oral risperidone prior to initiating treatment with INVEGA® SUSTENNA®.
Previous oral antipsychotics can be discontinued at the time of initiation of treatment with INVEGA® SUSTENNA®. Recommended initiation of INVEGA® SUSTENNA® is with a dose of 234 mg on treatment day 1 and 156 mg one week later, both administered in the deltoid muscle [see DOSAGE AND ADMINISTRATION (2.2)]. Patients previously stabilized on different doses of INVEGA® Extended-Release tablets can attain similar paliperidone steady-state exposure during maintenance treatment with INVEGA® SUSTENNA® monthly doses as depicted in Table 4.
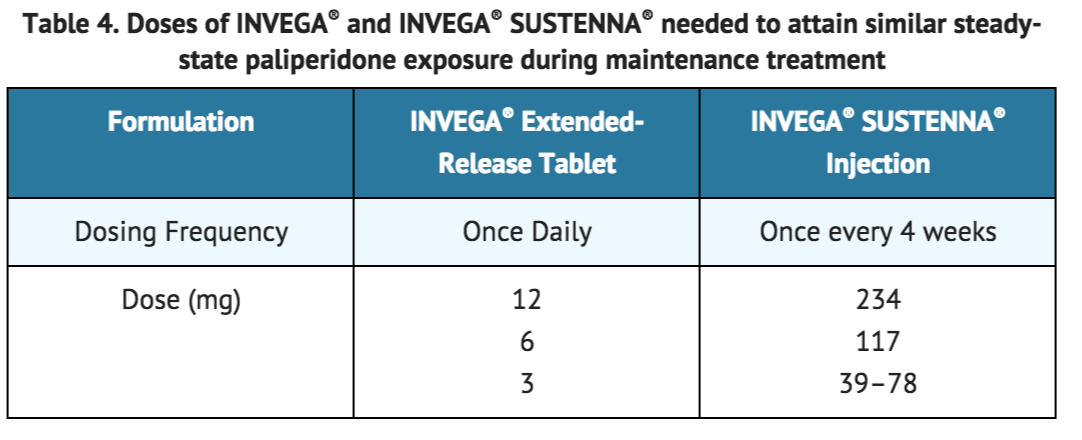
2.6.2 Switching from Long-Acting Injectable Antipsychotics
For patients who have never taken oral paliperidone or oral or injectable risperidone, tolerability should be established with oral paliperidone or oral risperidone prior to initiating treatment with INVEGA® SUSTENNA®.
When switching patients currently at steady-state on a long-acting injectable antipsychotic, initiate INVEGA® SUSTENNA® therapy in place of the next scheduled injection. INVEGA® SUSTENNA® should then be continued at monthly intervals. The one-week initiation dosing regimen as described in Section 2.2 is not required. See TABLE 1 above for recommended monthly maintenance dosing. Based on previous clinical history of tolerability and/or efficacy, some patients may benefit from lower or higher maintenance doses within the available strengths (39 mg, 78 mg, 117 mg, 156 mg, and 234 mg). The 39 mg strength was not studied in the long-term schizoaffective disorder study. Monthly maintenance doses can be administered in either the deltoid or gluteal muscle [see DOSAGE AND ADMINISTRATION (2.2)].
If INVEGA® SUSTENNA® is discontinued, its prolonged-release characteristics must be considered. As recommended with other antipsychotic medications, the need for continuing existing extrapyramidal symptoms (EPS) medication should be re-evaluated periodically.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Paliperidone (injection) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Paliperidone (injection) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Paliperidone (injection) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Paliperidone (injection) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Paliperidone (injection) in pediatric patients.
Contraindications
There is limited information regarding Paliperidone (injection) Contraindications in the drug label.
Warnings
|
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
See full prescribing information for complete Boxed Warning.
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death [see WARNINGS AND PRECAUTIONS (5.1)].
INVEGA® SUSTENNA® is not approved for use in patients with dementia-related psychosis [see WARNINGS AND PRECAUTIONS (5.1)].
|
There is limited information regarding Paliperidone (injection) Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Paliperidone (injection) Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Paliperidone (injection) Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Paliperidone (injection) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Paliperidone (injection) in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Paliperidone (injection) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Paliperidone (injection) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Paliperidone (injection) in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Paliperidone (injection) in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Paliperidone (injection) in geriatric settings.
Gender
There is no FDA guidance on the use of Paliperidone (injection) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Paliperidone (injection) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Paliperidone (injection) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Paliperidone (injection) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Paliperidone (injection) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Paliperidone (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Paliperidone (injection) Administration in the drug label.
Monitoring
There is limited information regarding Paliperidone (injection) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Paliperidone (injection) and IV administrations.
Overdosage
There is limited information regarding Paliperidone (injection) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Paliperidone (injection) Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Paliperidone (injection) Mechanism of Action in the drug label.
Structure
There is limited information regarding Paliperidone (injection) Structure in the drug label.
Pharmacodynamics
There is limited information regarding Paliperidone (injection) Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Paliperidone (injection) Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Paliperidone (injection) Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Paliperidone (injection) Clinical Studies in the drug label.
How Supplied
There is limited information regarding Paliperidone (injection) How Supplied in the drug label.
Storage
There is limited information regarding Paliperidone (injection) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Paliperidone (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Paliperidone (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Paliperidone (injection) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Paliperidone (injection) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Paliperidone (injection) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Paliperidone (injection) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.