Nystatin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Deepika Beereddy, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Nystatin is a {{{drugClass}}} that is FDA approved for the {{{indicationType}}} of candidal vulvovaginitis, candidiasis of skin, cutaneous and mucocutaneous infections, gastrointestinal candidiasis(non-esophageal), oropharyngeal candidiasis. Common adverse reactions include skin irritation, hypersensitivity reaction.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Nystatin Cream USP is indicated in the treatment of cutaneous or mucocutaneous mycotic infections caused by Candida albicans and other susceptible Candida species.
Nystatin Cream USP is not indicated for systemic, oral, intravaginal or ophthalmic use.
Candidal vulvovaginitis
- Dosing Information
- 1 tablet (100,000 units) INTRAVAGINALLY daily for 2 weeks.
Candidiasis of skin, Cutaneous and mucocutaneous infections
- Dosing Information
- ointment or cream, apply liberally to affected areas TOPICALLY twice daily until healing complete.
- powder, apply to candidal lesions TOPICALLY 2 to 3 times daily until healing complete; for fungal infections of the feet, footwear should be dusted as well.
Gastrointestinal candidiasis, Non-esophageal
- Dosing Information
- Tablet, 1 to 2 tablets (500,000 to 1,000,000 units) ORALLY 3 times per day; continue treatment for at least 48 hr after clinical cure
Oropharyngeal candidiasis
- Dosing Information
- Oral suspension, 4 to 6 mL (400,000 to 600,000 units) ORALLY (retained in mouth as long as possible prior to swallowing) 4 times daily; continue treatment for at least 48 hr after perioral symptoms disappear
- (HIV patients, duration): treat for 7 to 14 days (guideline dosing).
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Nystatin in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Nystatin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Candidiasis of skin, Cutaneous and mucocutaneous infections
- Dosing Information
- Ointment or cream, apply liberally to affected areas TOPICALLY twice daily until healing complete.
- Powder, apply to candidal lesions TOPICALLY 2 to 3 times daily until healing complete; for fungal infections of the feet, footwear should be dusted as well.
Oropharyngeal candidiasis
- Oral suspension, infants; 2 mL (200,000 units) ORALLY 4 times/day; continue treatment for at least 48 hr after perioral symptoms disappear.
- Oral suspension, children; 4 to 6 mL (400,000 to 600,000 units) ORALLY (retained in mouth as long as possible prior to swallowing) 4 times daily; continue treatment for at least 48 hr after perioral symptoms disappear.
- (HIV patients, duration) treat for 7 to 14 days (guideline dosing).
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Nystatin in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Nystatin in pediatric patients.
Contraindications
- Nystatin Cream USP is contraindicated in patients with a history of hypersensitivity to any of its components.
Warnings
Precautions
- General -
Nystatin Cream USP should not be used for the treatment of systemic, oral, intravaginal or ophthalmic infections.
If irritation or sensitization develops, treatment should be discontinued and appropriate measures taken as indicated. It is recommended that KOH smears, cultures, or other diagnostic methods be used to confirm the diagnosis of cutaneous or mucocutaneous candidiasis and to rule out infection caused by other pathogens.
Information for Patients -
Patients using this medication should receive the following information and instructions:
1. The patient should be instructed to use this medication as directed (including the replacement of missed doses). This medication is not for any disorder other than that for which it is prescribed.
2. Even if symptomatic relief occurs within the first few days of treatment, the patient should be advised not to interrupt or discontinue therapy until the prescribed course of treatment is completed.
3. If symptoms of irritation develop, the patient should be advised to notify the physician promptly.
Laboratory Tests -
If there is a lack of therapeutic response, KOH smears, cultures, or other diagnostic methods should be repeated.
Carcinogenesis, Mutagenesis, Impairment of Fertility -
No long-term animal studies have been performed to evaluate the carcinogenic potential of Nystatin. No studies have been performed to determine the mutagenicity of Nystatin or its effects on male or female fertility.
Pregnancy:
Teratogenic Effects:
Category C -
Animal reproduction studies have not been conducted with any Nystatin topical preparation. It also is not known whether these preparations can cause fetal harm when used by a pregnant woman or can affect reproductive capacity. Nystatin topical preparations should be prescribed for a pregnant woman only if the potential benefit to the mother outweighs the potential risk to the fetus.
Nursing Mothers -
It is not known whether Nystatin is excreted in human milk. Caution should be exercised when nystatin is prescribed for a nursing woman.
Pediatric Use -
Safety and effectiveness have been established in the pediatric population from birth to 16 years (see DOSAGE AND ADMINISTRATION).
Geriatric Use -
Clinical studies with nystatin cream did not include sufficient numbers of subjects aged 65 years and older to determine whether they respond differently than younger subjects. Other reported clinical experience has not identified differences in responses between elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Adverse Reactions
Clinical Trials Experience
The frequency of adverse events reported in patients using Nystatin Cream USP is less than 0.1%. The more common events that were reported include allergic reactions, burning, itching, rash, eczema, and pain on application (see PRECAUTIONS-General).
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Nystatin in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Nystatin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Nystatin during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Nystatin with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Nystatin with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Nystatin with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Nystatin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Nystatin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Nystatin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Nystatin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Nystatin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Nystatin in patients who are immunocompromised.
Administration and Monitoring
Administration
Very moist lesions are best treated with nystatin topical dusting powder.
Adults and Pediatric Patients (Neonates and Older):
Apply liberally to affected areas twice daily or as indicated until healing is complete.
Monitoring
There is limited information regarding Monitoring of Nystatin in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Nystatin in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Nystatin in the drug label.
Pharmacology
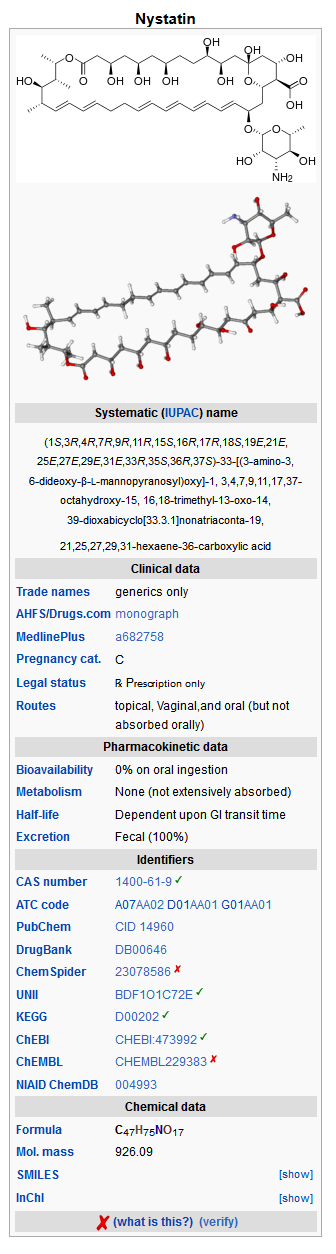
Mechanism of Action
Structure
Nystatin is a polyene antifungal antibiotic obtained from Streptomyces noursei. The molecular formula is C47H75NO17, and the molecular weight is 926.13.
Structural formula:
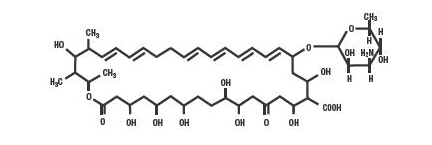
Nystatin Cream USP is for dermatologic use.
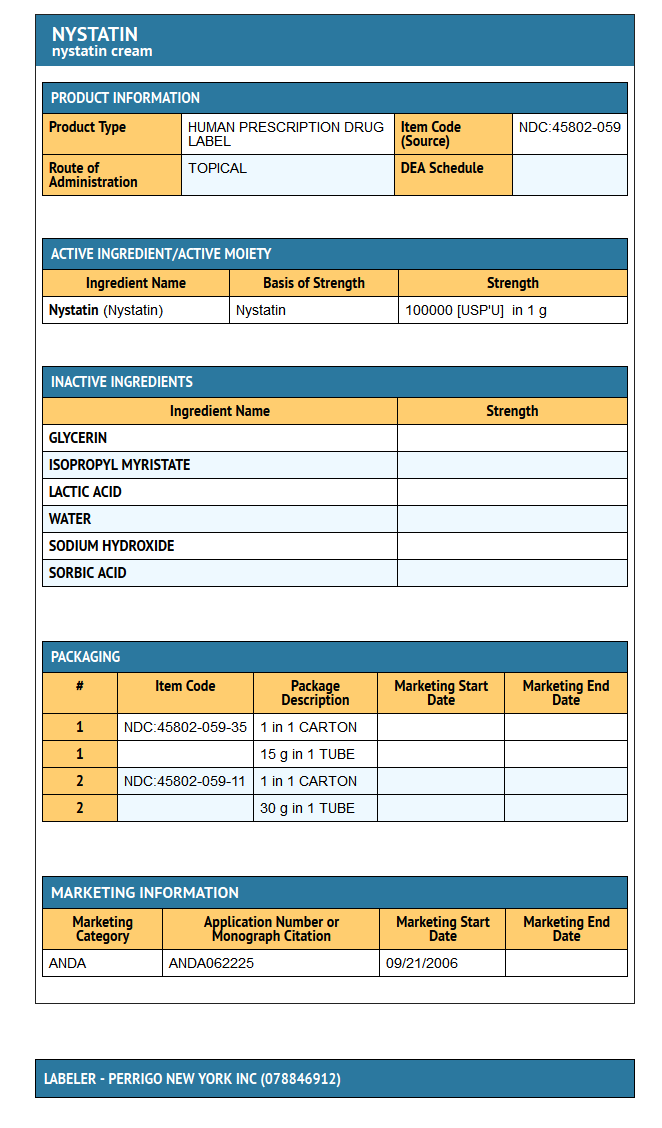
Nystatin Cream USP for topical use, contains 100,000 USP nystatin units per gram. Inactive ingredients: emulsifying wax, glycerin, isopropyl myristate, lactic acid, purified water, sodium hydroxide, and sorbic acid.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Nystatin in the drug label.
Pharmacokinetics
Nystatin Cream USP is not absorbed from intact skin or mucous membrane.
Microbiology
Nystatin is an antibiotic which is both fungistatic and fungicidal in vitro against a wide variety of yeasts and yeast-like fungi, including Candida albicans, C. parapsilosis, C. tropicalis, C. guilliermondi, C. pseudotropicalis, C. krusei, Torulopsisglabrata, Tricophytonrubrum, T. mentagrophytes.
Nystatin acts by binding to sterols in the cell membrane of susceptible species resulting in a change in membrane permeability and the subsequent leakage of intracellular components. On repeated subculturing with increasing levels of nystatin, Candida albicans does not develop resistance to nystatin. Generally, resistance to nystatin does not develop during therapy. However, other species of Candida (C. tropicalis, C. guilliermondi, C. krusei, and C. stellatoides) become quite resistant on treatment with nystatin and simultaneously become cross resistant to amphotericin as well. This resistance is lost when the antibiotic is removed.
Nystatin exhibits no appreciable activity against bacteria, protozoa, or viruses.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Nystatin in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Nystatin in the drug label.
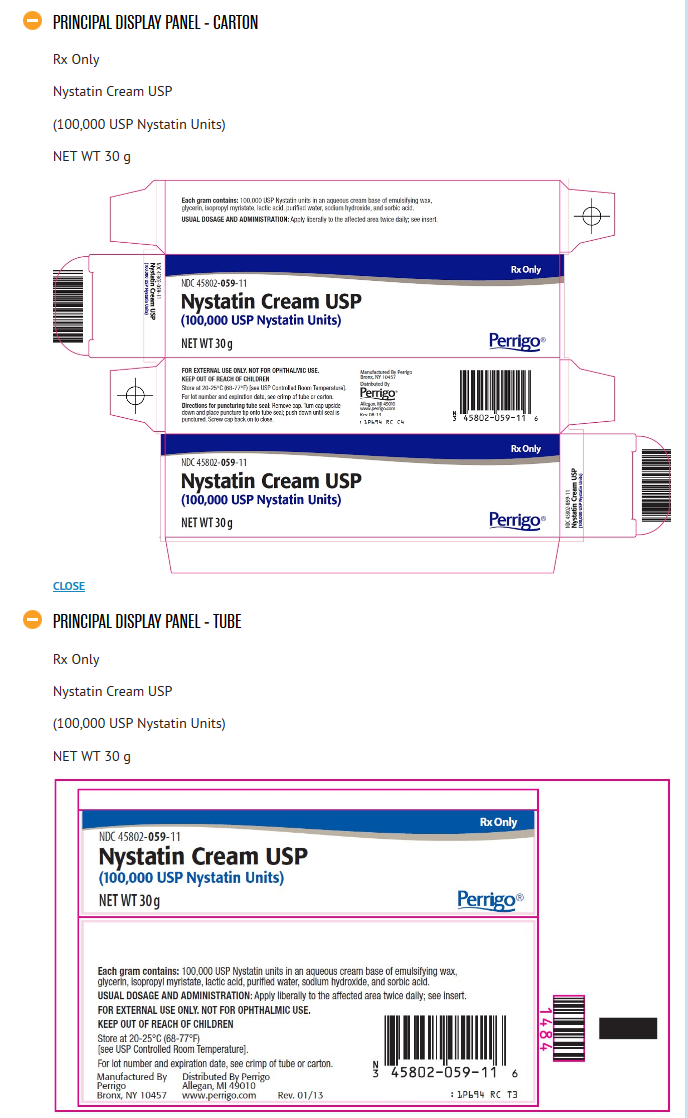
How Supplied
Nystatin Cream USP (100,000 USP Nystatin Units per gram) is a yellow cream available as follows:
15 g tube (NDC 45802-059-35)
30 g tube (NDC 45802-059-11)
Storage
Store at 20-25°C (68-77°F) [see USP Controlled Room Temperature].
Images
Drug Images
{{#ask: Page Name::Nystatin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Nystatin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Nystatin in the drug label.
Precautions with Alcohol
- Alcohol-Nystatin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Nystatin
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Nystatin |Label Name=Nystatin11.png
}}
{{#subobject:
|Label Page=Nystatin |Label Name=Nystatin11.png
}}