Netarsudil: Difference between revisions
No edit summary |
No edit summary |
||
| Line 10: | Line 10: | ||
|fdaLIADAdult======Indications:===== | |fdaLIADAdult======Indications:===== | ||
* | *Netarsudil 0.02% is indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension. | ||
=====Dosage:===== | =====Dosage:===== | ||
| Line 16: | Line 16: | ||
*The recommended dosage is one drop in the affected eye(s) once daily in the evening. | *The recommended dosage is one drop in the affected eye(s) once daily in the evening. | ||
*If one dose is missed, treatment should continue with the next dose in the evening. Twice a day dosing is not well tolerated and is not recommended. If | *If one dose is missed, treatment should continue with the next dose in the evening. Twice a day dosing is not well tolerated and is not recommended. If Netarsudil is to be used concomitantly with other topical ophthalmic drug products to lower IOP, administer each drug product at least 5 minutes apart. | ||
|offLabelAdultGuideSupport=There is limited information regarding Netarsudil ''Off-Label Guideline-Supported Use and Dosage (Adult)'' in the drug label. | |offLabelAdultGuideSupport=There is limited information regarding Netarsudil ''Off-Label Guideline-Supported Use and Dosage (Adult)'' in the drug label. | ||
| Line 36: | Line 36: | ||
=====Use with Contact Lenses===== | =====Use with Contact Lenses===== | ||
*Contact lenses should be removed prior to instillation of | *Contact lenses should be removed prior to instillation of Netarsudil and may be reinserted 15 minutes following its administration. | ||
|clinicalTrials=*Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice. | |clinicalTrials=*Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice. | ||
*The most common ocular adverse reaction observed in controlled clinical studies with | *The most common ocular adverse reaction observed in controlled clinical studies with Netarsudil dosed once daily was conjunctival hyperemia which was reported in 53% of patients. Other common (approximately 20%) ocular adverse reactions reported were: corneal verticillata, instillation site pain, and conjunctival hemorrhage. Instillation site erythema, corneal staining, blurred vision, increased lacrimation, erythema of eyelid, and reduced visual acuity were reported in 5-10% of patients. | ||
Corneal Verticillata | Corneal Verticillata | ||
*Corneal verticillata occurred in approximately 20% of the patients in controlled clinical studies. The corneal verticillata seen in | *Corneal verticillata occurred in approximately 20% of the patients in controlled clinical studies. The corneal verticillata seen in Netarsudil-treated patients were first noted at 4 weeks of daily dosing. This reaction did not result in any apparent visual functional changes in patients. Most corneal verticillata resolved upon discontinuation of treatment. | ||
|postmarketing= | |postmarketing= | ||
| Line 52: | Line 52: | ||
|useInPregnancyFDA=Risk Summary | |useInPregnancyFDA=Risk Summary | ||
*There are no available data on | *There are no available data on Netarsudil use in pregnant women to inform any drug associated risk; however, systemic exposure to Netarsudil from ocular administration is low. Intravenous administration of Netarsudil to pregnant rats and rabbits during organogenesis did not produce adverse embryofetal effects at clinically relevant systemic exposures. | ||
Data (Animal) | Data (Animal) | ||
| Line 63: | Line 63: | ||
|useInNursing=Risk Summary | |useInNursing=Risk Summary | ||
*There are no data on the presence of | *There are no data on the presence of Netarsudil in human milk, the effects on the breastfed infant, or the effects on milk production. However, systemic exposure to Netarsudil following topical ocular administration is low, and it is not known whether measurable levels of Netarsudil would be present in maternal milk following topical ocular administration. | ||
*The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for | *The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Netarsudil and any potential adverse effects on the breast-fed child from Netarsudil. | ||
|useInPed=*Safety and effectiveness in pediatric patients below the age of 18 years have not been established. | |useInPed=*Safety and effectiveness in pediatric patients below the age of 18 years have not been established. | ||
| Line 151: | Line 151: | ||
|PK=Absorption | |PK=Absorption | ||
*The systemic exposures of | *The systemic exposures of Netarsudil and its active metabolite, AR-13503, were evaluated in 18 healthy subjects after topical ocular administration of Netarsudil 0.02% once daily (one drop bilaterally in the morning) for 8 days. There were no quantifiable plasma concentrations of Netarsudil (lower limit of quantitation (LLOQ) 0.100 ng/mL) post dose on Day 1 and Day 8. Only one plasma concentration at 0.11 ng/mL for the active metabolite was observed for one subject on Day 8 at 8 hours post-dose. | ||
Metabolism | Metabolism | ||
*After topical ocular dosing, | *After topical ocular dosing, Netarsudil is metabolized by esterases in the eye. | ||
|nonClinToxic======Carcinogenesis, Mutagenesis, Impairment of Fertility===== | |nonClinToxic======Carcinogenesis, Mutagenesis, Impairment of Fertility===== | ||
*Long-term studies in animals have not been performed to evaluate the carcinogenic potential of | *Long-term studies in animals have not been performed to evaluate the carcinogenic potential of Netarsudil. Netarsudil was not mutagenic in the Ames test, in the mouse lymphoma test, or in the in vivo rat micronucleus test. Studies to evaluate the effects of Netarsudil on male or female fertility in animals have not been performed. | ||
|clinicalStudies=* | |clinicalStudies=* Netarsudil 0.02% was evaluated in three randomized and controlled clinical trials, namely AR-13324-CS301 (NCT 02207491, referred to as Study 301), AR-13324-CS302 (NCT 02207621, referred to as Study 302), and AR-13324-CS304 (NCT 02558374, referred to as Study 304), in patients with open-angle glaucoma or ocular hypertension. Studies 301 and 302 enrolled subjects with baseline IOP lower than 27 mmHg and Study 304 enrolled subjects with baseline IOP lower than 30 mmHg. The treatment duration was 3 months in Study 301, 12 months in Study 302, and 6 months in Study 304. | ||
*The three studies demonstrated up to 5 mmHg reductions in IOP for subjects treated with | *The three studies demonstrated up to 5 mmHg reductions in IOP for subjects treated with Netarsudil 0.02% once daily in the evening. For patients with baseline IOP < 25 mmHg, the IOP reductions with Netarsudil 0.02% dosed once daily were similar to those with timolol 0.5% dosed twice daily (see Table 1). For patients with baseline IOP equal to or above 25 mmHg, however, Netarsudil 0.02% resulted in smaller mean IOP reductions at the morning time points than timolol 0.5% for study visits on Days 43 and 90; the difference in mean IOP reduction between the two treatment groups was as high as 3 mmHg, favoring timolol. | ||
[[image:Netarsudil_Clinical_Studies_Table.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | [[image:Netarsudil_Clinical_Studies_Table.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | ||
*This table was produced based on the observed data from all randomized subjects who did not have major protocol violations. The treatment differences and two-sided CIs for comparing | *This table was produced based on the observed data from all randomized subjects who did not have major protocol violations. The treatment differences and two-sided CIs for comparing Netarsudil QD vs Timolol BID 0.5% were based on Analysis of Covariance (ANCOVA) adjusted for baseline IOP. | ||
|howSupplied= | |howSupplied=Netarsudil 0.02% (0.2 mg per mL) is supplied sterile in opaque white low density polyethylene bottles and tips with white polypropylene caps. | ||
:*2.5 mL fill in a 4 mL container | :*2.5 mL fill in a 4 mL container | ||
| Line 187: | Line 187: | ||
When to Seek Physician Advice | When to Seek Physician Advice | ||
*Advise patients that if they develop an intercurrent ocular condition (e.g., trauma or infection), have ocular surgery, or develop any ocular reactions, particularly conjunctivitis and eyelid reactions, they should immediately seek their physician’s advice concerning the continued use of | *Advise patients that if they develop an intercurrent ocular condition (e.g., trauma or infection), have ocular surgery, or develop any ocular reactions, particularly conjunctivitis and eyelid reactions, they should immediately seek their physician’s advice concerning the continued use of Netarsudil. | ||
Use with Contact Lenses | Use with Contact Lenses | ||
*Advise patients that | *Advise patients that Netarsudil contains benzalkonium chloride, which may be absorbed by soft contact lenses. Contact lenses should be removed prior to instillation of Netarsudil and may be reinserted 15 minutes following its administration. | ||
Use with Other Ophthalmic Drugs | Use with Other Ophthalmic Drugs | ||
Latest revision as of 17:22, 20 July 2018
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Yashasvi Aryaputra[2];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Netarsudil is a Rho kinase inhibitor that is FDA approved for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. Common adverse reactions include conjunctival hyperemia, corneal verticillata, instillation site pain, and conjunctival hemorrhage.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications:
- Netarsudil 0.02% is indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension.
Dosage:
- The recommended dosage is one drop in the affected eye(s) once daily in the evening.
- If one dose is missed, treatment should continue with the next dose in the evening. Twice a day dosing is not well tolerated and is not recommended. If Netarsudil is to be used concomitantly with other topical ophthalmic drug products to lower IOP, administer each drug product at least 5 minutes apart.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Netarsudil Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding Netarsudil Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Netarsudil FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Netarsudil Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding Netarsudil Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
- None
Warnings
Bacterial Keratitis
- There have been reports of bacterial keratitis associated with the use of multiple-dose containers of topical ophthalmic products. These containers had been inadvertently contaminated by patients who, in most cases, had a concurrent corneal disease or a disruption of the ocular epithelial surface.
Use with Contact Lenses
- Contact lenses should be removed prior to instillation of Netarsudil and may be reinserted 15 minutes following its administration.
Adverse Reactions
Clinical Trials Experience
- Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
- The most common ocular adverse reaction observed in controlled clinical studies with Netarsudil dosed once daily was conjunctival hyperemia which was reported in 53% of patients. Other common (approximately 20%) ocular adverse reactions reported were: corneal verticillata, instillation site pain, and conjunctival hemorrhage. Instillation site erythema, corneal staining, blurred vision, increased lacrimation, erythema of eyelid, and reduced visual acuity were reported in 5-10% of patients.
Corneal Verticillata
- Corneal verticillata occurred in approximately 20% of the patients in controlled clinical studies. The corneal verticillata seen in Netarsudil-treated patients were first noted at 4 weeks of daily dosing. This reaction did not result in any apparent visual functional changes in patients. Most corneal verticillata resolved upon discontinuation of treatment.
Postmarketing Experience
There is limited information regarding Netarsudil Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Netarsudil Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Risk Summary
- There are no available data on Netarsudil use in pregnant women to inform any drug associated risk; however, systemic exposure to Netarsudil from ocular administration is low. Intravenous administration of Netarsudil to pregnant rats and rabbits during organogenesis did not produce adverse embryofetal effects at clinically relevant systemic exposures.
Data (Animal)
- Netarsudil administered daily by intravenous injection to rats during organogenesis caused abortions and embryofetal lethality at doses ≥0.3 mg/kg/day (126-fold the plasma exposure at the recommended human ophthalmic dose [RHOD], based on Cmax). The no-observed-adverse-effect-level (NOAEL) for embryofetal development toxicity was 0.1 mg/kg/day (40-fold the plasma exposure at the RHOD, based on Cmax).
- Netarsudil administered daily by intravenous injection to rabbits during organogenesis caused embryofetal lethality and decreased fetal weight at 5 mg/kg/day (1480-fold the plasma exposure at the RHOD, based on Cmax). Malformations were observed at ≥3 mg/kg/day (1330-fold the plasma exposure at the RHOD, based on Cmax), including thoracogastroschisis, umbilical hernia and absent intermediate lung lobe. The NOAEL for embryofetal development toxicity was 0.5 mg/kg/day (214-fold the plasma exposure at the RHOD, based on Cmax).
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Netarsudil in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Netarsudil during labor and delivery.
Nursing Mothers
Risk Summary
- There are no data on the presence of Netarsudil in human milk, the effects on the breastfed infant, or the effects on milk production. However, systemic exposure to Netarsudil following topical ocular administration is low, and it is not known whether measurable levels of Netarsudil would be present in maternal milk following topical ocular administration.
- The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Netarsudil and any potential adverse effects on the breast-fed child from Netarsudil.
Pediatric Use
- Safety and effectiveness in pediatric patients below the age of 18 years have not been established.
Geriatic Use
- No overall differences in safety or effectiveness have been observed between elderly and other adult patients.
Gender
There is no FDA guidance on the use of Netarsudil with respect to specific gender populations.
Race
There is no FDA guidance on the use of Netarsudil with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Netarsudil in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Netarsudil in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Netarsudil in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Netarsudil in patients who are immunocompromised.
Administration and Monitoring
Administration
Ophthalmic
- Administer in the evening.
- When used concomitantly with other ophthalmic products intended to lower intraocular pressure, separate administration of each product by at least 5 minutes.
Monitoring
- A reduction in intraocular pressure indicates efficacy.
IV Compatibility
There is limited information regarding the compatibility of Netarsudil and IV administrations.
Overdosage
There is limited information regarding Netarsudil overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
| |
Netarsudil
| |
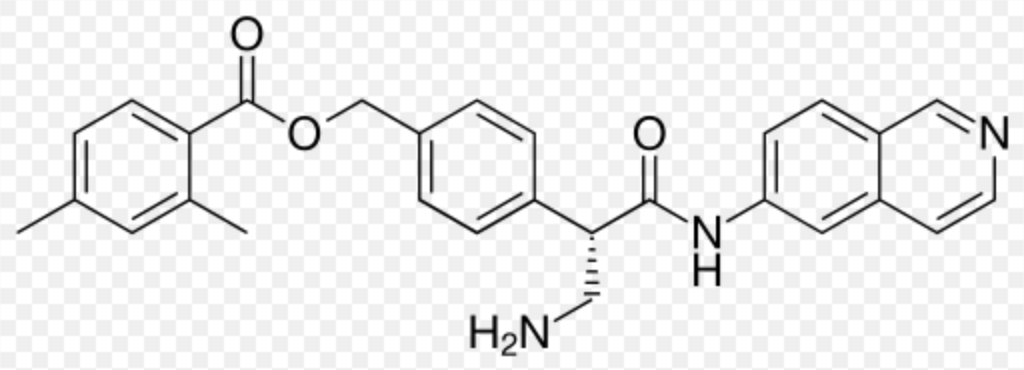
| Systematic (IUPAC) name | |
| [4-[(2S)-3-Amino-1-(isoquinolin-6-ylamino)-1-oxopropan-2-yl]phenyl]methyl 2,4-dimethylbenzoate | |
| Identifiers | |
| CAS number | |
| ATC code | ? |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | ? |
| Synonyms | AR-11324 |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | ? |
Mechanism of Action
- Netarsudil is a rho kinase inhibitor, which is believed to reduce IOP by increasing the outflow of aqueous humor through the trabecular meshwork route. The exact mechanism is unknown.
Structure
Pharmacodynamics
There is limited information regarding Netarsudil Pharmacodynamics in the drug label.
Pharmacokinetics
Absorption
- The systemic exposures of Netarsudil and its active metabolite, AR-13503, were evaluated in 18 healthy subjects after topical ocular administration of Netarsudil 0.02% once daily (one drop bilaterally in the morning) for 8 days. There were no quantifiable plasma concentrations of Netarsudil (lower limit of quantitation (LLOQ) 0.100 ng/mL) post dose on Day 1 and Day 8. Only one plasma concentration at 0.11 ng/mL for the active metabolite was observed for one subject on Day 8 at 8 hours post-dose.
Metabolism
- After topical ocular dosing, Netarsudil is metabolized by esterases in the eye.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Long-term studies in animals have not been performed to evaluate the carcinogenic potential of Netarsudil. Netarsudil was not mutagenic in the Ames test, in the mouse lymphoma test, or in the in vivo rat micronucleus test. Studies to evaluate the effects of Netarsudil on male or female fertility in animals have not been performed.
Clinical Studies
- Netarsudil 0.02% was evaluated in three randomized and controlled clinical trials, namely AR-13324-CS301 (NCT 02207491, referred to as Study 301), AR-13324-CS302 (NCT 02207621, referred to as Study 302), and AR-13324-CS304 (NCT 02558374, referred to as Study 304), in patients with open-angle glaucoma or ocular hypertension. Studies 301 and 302 enrolled subjects with baseline IOP lower than 27 mmHg and Study 304 enrolled subjects with baseline IOP lower than 30 mmHg. The treatment duration was 3 months in Study 301, 12 months in Study 302, and 6 months in Study 304.
- The three studies demonstrated up to 5 mmHg reductions in IOP for subjects treated with Netarsudil 0.02% once daily in the evening. For patients with baseline IOP < 25 mmHg, the IOP reductions with Netarsudil 0.02% dosed once daily were similar to those with timolol 0.5% dosed twice daily (see Table 1). For patients with baseline IOP equal to or above 25 mmHg, however, Netarsudil 0.02% resulted in smaller mean IOP reductions at the morning time points than timolol 0.5% for study visits on Days 43 and 90; the difference in mean IOP reduction between the two treatment groups was as high as 3 mmHg, favoring timolol.
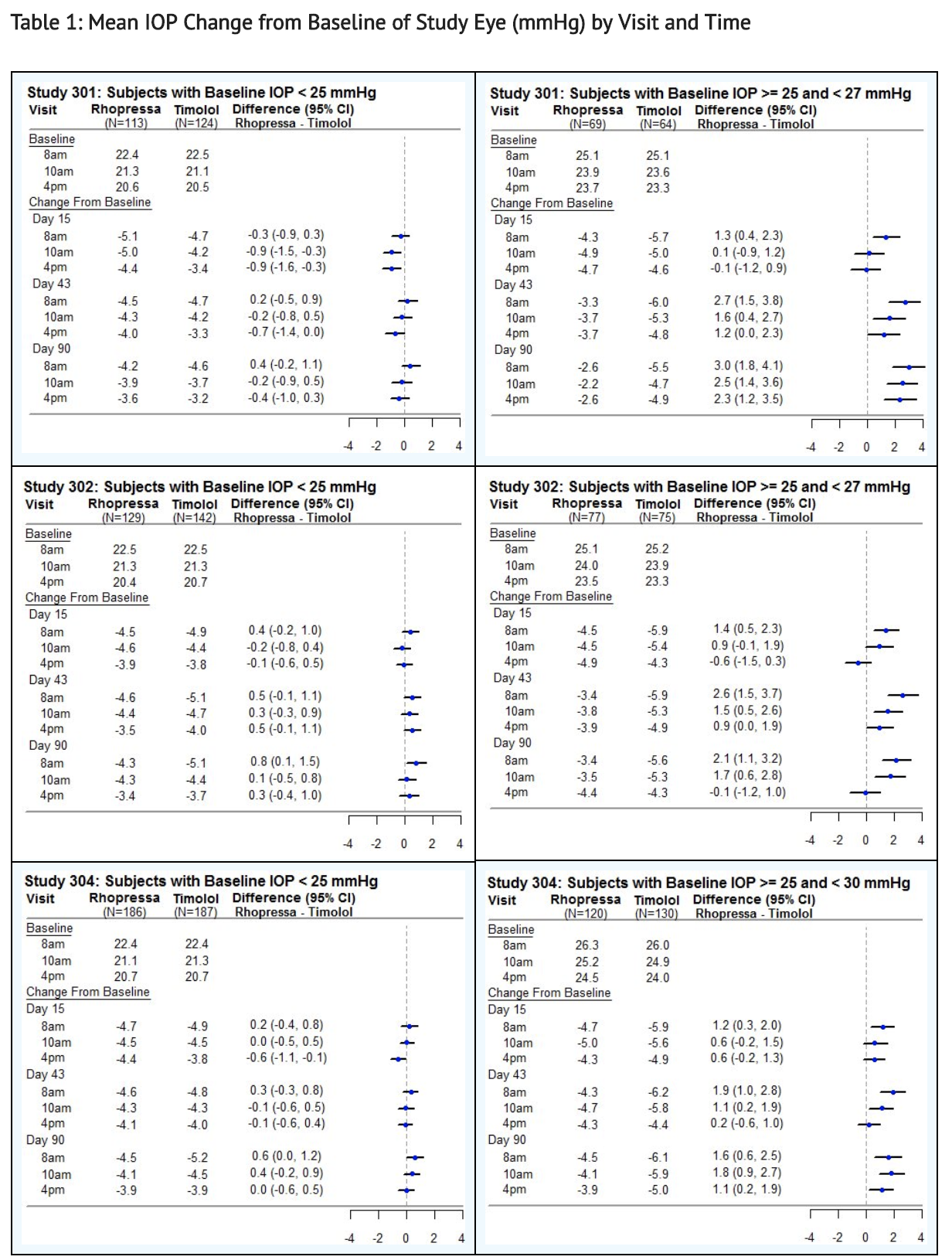
- This table was produced based on the observed data from all randomized subjects who did not have major protocol violations. The treatment differences and two-sided CIs for comparing Netarsudil QD vs Timolol BID 0.5% were based on Analysis of Covariance (ANCOVA) adjusted for baseline IOP.
How Supplied
Netarsudil 0.02% (0.2 mg per mL) is supplied sterile in opaque white low density polyethylene bottles and tips with white polypropylene caps.
- 2.5 mL fill in a 4 mL container
- NDC # 70727-497-25
Storage
- Storage: Store at 2°C to 8°C (36°F to 46°F) until opened. After opening, the product may be kept at 2°C to 25°C (36°F to 77°F) for up to 6 weeks. During shipment, the bottle may be maintained at temperatures up to 40°C (104°F) for a period not exceeding 14 days.
Images
Drug Images
{{#ask: Page Name::Netarsudil |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
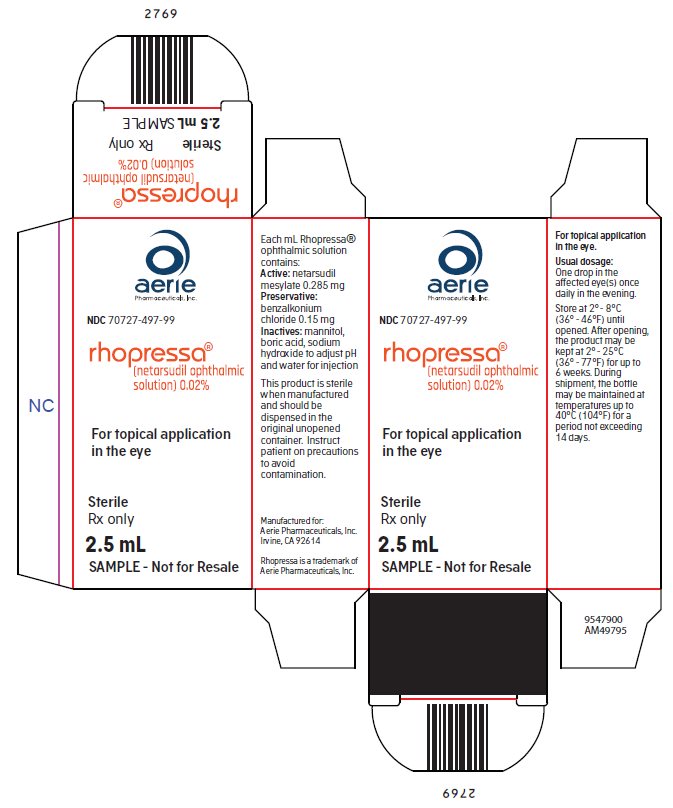
{{#ask: Label Page::Netarsudil |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Handling the Container
- Instruct patients to avoid allowing the tip of the dispensing container to contact the eye, surrounding structures, fingers, or any other surface in order to minimize contamination of the solution. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions.
When to Seek Physician Advice
- Advise patients that if they develop an intercurrent ocular condition (e.g., trauma or infection), have ocular surgery, or develop any ocular reactions, particularly conjunctivitis and eyelid reactions, they should immediately seek their physician’s advice concerning the continued use of Netarsudil.
Use with Contact Lenses
- Advise patients that Netarsudil contains benzalkonium chloride, which may be absorbed by soft contact lenses. Contact lenses should be removed prior to instillation of Netarsudil and may be reinserted 15 minutes following its administration.
Use with Other Ophthalmic Drugs
- Advise patients that if more than one topical ophthalmic drug is being used, the drugs should be administered at least 5 minutes between applications.
Missed Dose
- Advise patients that if one dose is missed, treatment should continue with the next dose in the evening.
Precautions with Alcohol
Alcohol-Netarsudil interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Rhopressa
Look-Alike Drug Names
There is limited information regarding Netarsudil Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.