Neratinib: Difference between revisions
No edit summary |
No edit summary |
||
| Line 9: | Line 9: | ||
|fdaLIADAdult= | |fdaLIADAdult= | ||
=====Indication===== | =====Indication===== | ||
* | *Neratinib is indicated for the extended adjuvant treatment of adult patients with early stage HER2-overexpressed/amplified breast cancer, to follow adjuvant trastuzumab based therapy. | ||
=====Antidiarrheal Prophylaxis===== | =====Antidiarrheal Prophylaxis===== | ||
*Antidiarrheal prophylaxis is recommended during the first 2 cycles (56 days) of treatment and should be initiated with the first dose of | *Antidiarrheal prophylaxis is recommended during the first 2 cycles (56 days) of treatment and should be initiated with the first dose of neratinib. | ||
*Instruct patients to take loperamide as directed in TABLE 1, titrating to 1-2 bowel movements per day. | *Instruct patients to take loperamide as directed in TABLE 1, titrating to 1-2 bowel movements per day. | ||
[[image:neratinibdosage1.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | [[image:neratinibdosage1.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | ||
*Additional antidiarrheal agents may be required to manage diarrhea in patients with loperamide-refractory diarrhea. | *Additional antidiarrheal agents may be required to manage diarrhea in patients with loperamide-refractory diarrhea. Neratinib dose interruptions and dose reductions may also be required to manage diarrhea. | ||
=====Recommended Dose and Schedule===== | =====Recommended Dose and Schedule===== | ||
*The recommended dose of | *The recommended dose of neratinib is 240 mg (six tablets) given orally once daily with food, continuously for one year. | ||
*Instruct patients to take | *Instruct patients to take neratinib at approximately the same time every day. Neratinib tablets should be swallowed whole (tablets should not be chewed, crushed, or split prior to swallowing). | ||
*If a patient misses a dose, do not replace missed dose, and instruct the patient to resume | *If a patient misses a dose, do not replace missed dose, and instruct the patient to resume neratinib with the next scheduled daily dose. | ||
=====Dose Modifications===== | =====Dose Modifications===== | ||
=====Dose Modifications for Adverse Reactions===== | =====Dose Modifications for Adverse Reactions===== | ||
* | *Neratinib dose modification is recommended based on individual safety and tolerability. Management of some adverse reactions may require dose interruption and/or dose reduction as shown in TABLE 2 to TABLE 5. Discontinue neratinib for patients who fail to recover to Grade 0-1 from treatment-related toxicity, for toxicities that result in a treatment delay > 3 weeks, or for patients that are unable to tolerate 120 mg daily. Additional clinical situations may result in dose adjustments as clinically indicated (e.g. intolerable toxicities, persistent Grade 2 adverse reactions, etc.). | ||
[[image:neratinibdosage2.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | [[image:neratinibdosage2.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | ||
=====Dose Modifications for Diarrhea===== | =====Dose Modifications for Diarrhea===== | ||
*Diarrhea management requires the correct use of antidiarrheal medication, dietary changes, and appropriate dose modifications of | *Diarrhea management requires the correct use of antidiarrheal medication, dietary changes, and appropriate dose modifications of neratinib. Guidelines for adjusting doses of neratinib in the setting of diarrhea are shown in TABLE 4. | ||
[[image:neratinibdosage3.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | [[image:neratinibdosage3.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | ||
=====Dose Modifications for Hepatic Impairment===== | =====Dose Modifications for Hepatic Impairment===== | ||
*Reduce the | *Reduce the neratinib starting dose to 80 mg in patients with severe hepatic impairment (Child Pugh C). No dose modifications are recommended for patients with mild to moderate hepatic impairment (Child Pugh A or B). | ||
=====Dose Modifications for Hepatotoxicity===== | =====Dose Modifications for Hepatotoxicity===== | ||
*Guidelines for dose adjustment of | *Guidelines for dose adjustment of neratinib in the event of liver toxicity are shown in TABLE 5. Patients who experience ≥ Grade 3 diarrhea requiring IV fluid treatment or any signs or symptoms of hepatotoxicity, such as worsening of fatigue, nausea, vomiting, right upper quadrant pain or tenderness, fever, rash, or eosinophilia, should be evaluated for changes in liver function tests. Fractionated bilirubin and prothrombin time should also be collected during hepatotoxicity evaluation. | ||
[[image:neratinibdosage4.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | [[image:neratinibdosage4.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | ||
=====Concomitant Use with Gastric Acid Reducing Agents===== | =====Concomitant Use with Gastric Acid Reducing Agents===== | ||
*Proton pump inhibitors (PPI): Avoid concomitant use with | *Proton pump inhibitors (PPI): Avoid concomitant use with neratinib. | ||
*H2-receptor antagonists: Take | *H2-receptor antagonists: Take neratinib at least 2 hours before the next dose of the H 2-receptor antagonist or 10 hours after the H2-receptor antagonist. | ||
*Antacids: Separate dosing of | *Antacids: Separate dosing of neratinib by 3 hours after antacids. | ||
=====Dosage Forms and Strengths===== | =====Dosage Forms and Strengths===== | ||
*Tablets: 40 mg neratinib (equivalent to 48.31 mg of neratinib maleate). | *Tablets: 40 mg neratinib (equivalent to 48.31 mg of neratinib maleate). | ||
| Line 51: | Line 51: | ||
|warnings= | |warnings= | ||
=====Diarrhea===== | =====Diarrhea===== | ||
*Severe diarrhea and sequelae, such as dehydration, hypotension, and renal failure, have been reported during treatment with | *Severe diarrhea and sequelae, such as dehydration, hypotension, and renal failure, have been reported during treatment with neratinib. Diarrhea was reported in 95% of neratinib-treated patients in ExteNET, a randomized placebo controlled trial. In the neratinib arm, Grade 3 diarrhea occurred in 40% and Grade 4 diarrhea occurred in 0.1% of patients. The majority of patients (93%) had diarrhea in the first month of treatment, the median time to first onset of Grade ≥ 3 diarrhea was 8 days (range, 1-350), and the median cumulative duration of Grade ≥ 3 diarrhea was 5 days (range, 1-139). | ||
*Antidiarrheal prophylaxis with loperamide has been shown to lower the incidence and severity of diarrhea. Instruct patients to initiate antidiarrheal prophylaxis with loperamide along with the first dose of | *Antidiarrheal prophylaxis with loperamide has been shown to lower the incidence and severity of diarrhea. Instruct patients to initiate antidiarrheal prophylaxis with loperamide along with the first dose of neratinib and continue during the first two cycles (56 days) of treatment. | ||
*Monitor patients for diarrhea and treat with additional antidiarrheals as needed. When severe diarrhea with dehydration occurs, administer fluid and electrolytes as needed, interrupt | *Monitor patients for diarrhea and treat with additional antidiarrheals as needed. When severe diarrhea with dehydration occurs, administer fluid and electrolytes as needed, interrupt neratinib, and reduce subsequent doses. Perform stool cultures as clinically indicated to exclude infectious causes of Grade 3 or 4 diarrhea or diarrhea of any grade with complicating features (dehydration, fever, neutropenia). | ||
=====Hepatotoxicity===== | =====Hepatotoxicity===== | ||
* | *Neratinib has been associated with hepatotoxicity characterized by increased liver enzymes. In ExteNET, 9.7% of patients experienced an alanine aminotransferase (ALT) increase ≥ 2 x ULN, 5.1% of patients experienced an aspartate aminotransferase (AST) increase ≥ 2 x ULN, and 1.7% of patients experienced an AST or ALT elevation > 5 x ULN (≥ Grade 3). Hepatotoxicity or increases in liver transaminases led to drug discontinuation in 1.7% of neratinib-treated patients. | ||
*Total bilirubin, AST, ALT, and alkaline phosphatase should be measured prior to starting treatment with | *Total bilirubin, AST, ALT, and alkaline phosphatase should be measured prior to starting treatment with neratinib monthly for the first 3 months of treatment, then every 3 months while on treatment and as clinically indicated. These tests should also be performed in patients experiencing Grade 3 diarrhea or any signs or symptoms of hepatotoxicity, such as worsening of fatigue, nausea, vomiting, right upper quadrant tenderness, fever, rash, or eosinophilia. | ||
=====Embryo-Fetal Toxicity===== | =====Embryo-Fetal Toxicity===== | ||
*Based on findings from animal studies and its mechanism of action, | *Based on findings from animal studies and its mechanism of action, neratinib can cause fetal harm when administered to a pregnant woman. In animal reproduction studies, administration of neratinib to pregnant rabbits during organogenesis caused abortions, embryo-fetal death and fetal abnormalities in rabbits at maternal AUCs approximately 0.2 times the AUC in patients receiving the recommended dose. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment and for at least 1 month after the last dose. | ||
|clinicalTrials= | |clinicalTrials= | ||
*Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | *Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | ||
=====ExteNET===== | =====ExteNET===== | ||
*The data described below reflect exposure of | *The data described below reflect exposure of neratinib as a single agent in ExteNET, a multicenter, randomized, double-blind, placebo-controlled study of neratinib within 2 years after completion of adjuvant treatment with trastuzumab-based therapy in women with HER2-positive early-stage breast cancer. Patients who received neratinib in this trial were not required to receive any prophylaxis with antidiarrheal agents to prevent the neratinib-related diarrhea. The median duration of treatment was 11.6 months in the neratinib arm and 11.8 months in the placebo arm. The median age was 52 years (60% were ≥ 50 years old, 12% were ≥ 65 years old); 81% were Caucasian, 3% Black or African American, 14% Asian and 3% other. A total of 1408 patients were treated with neratinib. | ||
* | *Neratinib dose reduction due to an adverse reaction of any grade occurred in 31.2% of patients receiving neratinib compared to 2.6% of patients receiving placebo. Permanent discontinuation due to any adverse reaction was reported in 27.6% of neratinib-treated patients. The most common adverse reaction leading to discontinuation was diarrhea, accounting for 16.8% of neratinib-treated patients. | ||
*The most common adverse reactions (>5%) were diarrhea, nausea, abdominal pain, fatigue, vomiting, rash, stomatitis, decreased appetite, muscle spasms, dyspepsia, AST or ALT increase, nail disorder, dry skin, abdominal distention, weight decreased and urinary tract infection. The most frequently reported Grade 3 or 4 adverse reactions were diarrhea, vomiting, nausea, and abdominal pain. | *The most common adverse reactions (>5%) were diarrhea, nausea, abdominal pain, fatigue, vomiting, rash, stomatitis, decreased appetite, muscle spasms, dyspepsia, AST or ALT increase, nail disorder, dry skin, abdominal distention, weight decreased and urinary tract infection. The most frequently reported Grade 3 or 4 adverse reactions were diarrhea, vomiting, nausea, and abdominal pain. | ||
*Serious adverse reactions in the | *Serious adverse reactions in the neratinib arm included diarrhea (1.6%), vomiting (0.9%), dehydration (0.6%), cellulitis (0.4%), renal failure (0.4%), erysipelas (0.4%), alanine aminotransferase increased (0.3%), aspartate aminotransferase increased (0.3%), nausea (0.3%), fatigue (0.2%), and abdominal pain (0.2%). | ||
*TABLE 6 summarizes the adverse reactions in ExteNET. | *TABLE 6 summarizes the adverse reactions in ExteNET. | ||
[[image:neratinibclinexp.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | [[image:neratinibclinexp.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | ||
|postmarketing= | |postmarketing= | ||
|drugInteractions= | |drugInteractions= | ||
* Effect of Other Drugs on | * Effect of Other Drugs on Neratinib | ||
* Effect of | * Effect of Neratinib on Other Drugs | ||
=====Effect of Other Drugs on | =====Effect of Other Drugs on Neratinib===== | ||
*TABLE 7 includes drug interactions that affect the pharmacokinetics of neratinib. | *TABLE 7 includes drug interactions that affect the pharmacokinetics of neratinib. | ||
[[image:neratinibdrugint.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | [[image:neratinibdrugint.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | ||
=====Effect of | =====Effect of Neratinib on Other Drugs===== | ||
=====P-glycoprotein (P-gp) Substrates===== | =====P-glycoprotein (P-gp) Substrates===== | ||
*Concomitant use of | *Concomitant use of neratinib with digoxin, a P-gp substrate, increased digoxin concentrations. Increased concentrations of digoxin may lead to increased risk of adverse reactions including cardiac toxicity. Refer to the digoxin prescribing information for dosage adjustment recommendations due to drug interactions. neratinib may inhibit the transport of other P-gp substrates (e.g., dabigatran, fexofenadine). | ||
|useInPregnancyFDA= | |useInPregnancyFDA= | ||
=====Risk Summary===== | =====Risk Summary===== | ||
*Based on findings from animal studies and the mechanism of action, | *Based on findings from animal studies and the mechanism of action, neratinib can cause fetal harm when administered to a pregnant woman. | ||
*There are no available data in pregnant women to inform the drug-associated risk. In animal reproduction studies, administration of neratinib to pregnant rabbits during organogenesis resulted in abortions, embryo-fetal death and fetal abnormalities in rabbits at maternal exposures (AUC) approximately 0.2 times exposures in patients at the recommended dose. Advise pregnant women of the potential risk to a fetus. | *There are no available data in pregnant women to inform the drug-associated risk. In animal reproduction studies, administration of neratinib to pregnant rabbits during organogenesis resulted in abortions, embryo-fetal death and fetal abnormalities in rabbits at maternal exposures (AUC) approximately 0.2 times exposures in patients at the recommended dose. Advise pregnant women of the potential risk to a fetus. | ||
*The background risk of major birth defects and miscarriage for the indicated population is unknown. However, the background risk of major birth defects is 2-4% and of miscarriage is 15-20% of clinically recognized pregnancies in the U.S. general population. | *The background risk of major birth defects and miscarriage for the indicated population is unknown. However, the background risk of major birth defects is 2-4% and of miscarriage is 15-20% of clinically recognized pregnancies in the U.S. general population. | ||
| Line 91: | Line 91: | ||
|useInNursing= | |useInNursing= | ||
=====Risk Summary===== | =====Risk Summary===== | ||
*No data are available regarding the presence of neratinib or its metabolites in human milk or its effects on the breastfed infant or on milk production. Because of the potential for serious adverse reactions in breastfed infants from | *No data are available regarding the presence of neratinib or its metabolites in human milk or its effects on the breastfed infant or on milk production. Because of the potential for serious adverse reactions in breastfed infants from neratinib, advise lactating women not to breastfeed while taking neratinib and for at least 1 month after the last dose. | ||
|useInPed= | |useInPed= | ||
*The safety and efficacy of | *The safety and efficacy of neratinib in pediatric patients has not been established. | ||
|useInGeri= | |useInGeri= | ||
*In the ExteNET trial, the mean age was 52 years in the | *In the ExteNET trial, the mean age was 52 years in the neratinib arm; 1236 patients were < 65 years, 172 patients were ≥ 65 years, of whom 25 patients were 75 years or older. | ||
*There was a higher frequency of treatment discontinuations due to adverse reactions in the ≥ 65 years age group than in the < 65 years age group; in the | *There was a higher frequency of treatment discontinuations due to adverse reactions in the ≥ 65 years age group than in the < 65 years age group; in the neratinib arm, the percentages were 44.8% compared with 25.2%, respectively, and in the placebo arm 6.4% and 5.3%, respectively. | ||
*The incidence of serious adverse reactions in the | *The incidence of serious adverse reactions in the neratinib arm vs. placebo arm was 7.0% vs. 5.7% (< 65 years-old) and 9.9% vs. 8.1% (≥ 65 years-old). The serious adverse reactions most frequently reported in the ≥ 65 years-old group were vomiting (2.3%), diarrhea (1.7%), renal failure (1.7%), and dehydration (1.2%). | ||
|useInGender= | |useInGender= | ||
|useInRace= | |useInRace= | ||
|useInRenalImpair= | |useInRenalImpair= | ||
|useInHepaticImpair= | |useInHepaticImpair= | ||
*No dose modifications are recommended for patients with mild to moderate hepatic impairment (Child Pugh A or B). Patients with severe, pre-existing hepatic impairment (Child Pugh Class C) experienced a reduction in neratinib clearance and an increase in C<sub>max</sub> and AUC. Reduce the | *No dose modifications are recommended for patients with mild to moderate hepatic impairment (Child Pugh A or B). Patients with severe, pre-existing hepatic impairment (Child Pugh Class C) experienced a reduction in neratinib clearance and an increase in C<sub>max</sub> and AUC. Reduce the neratinib dosage for patients with severe hepatic impairment. | ||
|useInReproPotential= | |useInReproPotential= | ||
=====Pregnancy===== | =====Pregnancy===== | ||
*Based on animal studies, | *Based on animal studies, neratinib can cause fetal harm when administered to a pregnant woman. Females of reproductive potential should have a pregnancy test prior to starting treatment with neratinib. | ||
=====Contraception===== | =====Contraception===== | ||
''Females'' | ''Females'' | ||
*Based on animal studies, | *Based on animal studies, neratinib can cause fetal harm when administered to a pregnant woman. Advise females of reproductive potential to use effective contraception during treatment with neratinib and for at least 1 month after the last dose. | ||
''Males'' | ''Males'' | ||
*Based on findings in animal reproduction studies, advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 3 months after the last dose of | *Based on findings in animal reproduction studies, advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 3 months after the last dose of neratinib. | ||
|useInImmunocomp= | |useInImmunocomp= | ||
|administration= | |administration= | ||
| Line 122: | Line 122: | ||
*Pulmonary symptoms of interstitial lung disease or pneumonitis. | *Pulmonary symptoms of interstitial lung disease or pneumonitis. | ||
|overdose= | |overdose= | ||
*There is no specific antidote, and the benefit of hemodialysis in the treatment of | *There is no specific antidote, and the benefit of hemodialysis in the treatment of neratinib overdose is unknown. In the event of an overdose, administration should be withheld and general supportive measures undertaken. | ||
*In the clinical trial setting, a limited number of patients reported overdose. The adverse reactions experienced by these patients were diarrhea, nausea, vomiting, and dehydration. The frequency and severity of gastrointestinal disorders (diarrhea, abdominal pain, nausea and vomiting) appear to be dose related. | *In the clinical trial setting, a limited number of patients reported overdose. The adverse reactions experienced by these patients were diarrhea, nausea, vomiting, and dehydration. The frequency and severity of gastrointestinal disorders (diarrhea, abdominal pain, nausea and vomiting) appear to be dose related. | ||
|drugBox={{Drugbox2 | |drugBox={{Drugbox2 | ||
| Line 186: | Line 186: | ||
|PD= | |PD= | ||
=====Cardiac Electrophysiology===== | =====Cardiac Electrophysiology===== | ||
*The effect of | *The effect of neratinib on the QTc interval was evaluated in a randomized, placebo and positive controlled, double-blind, single-dose, crossover study in 60 healthy subjects. At 2.4-fold the therapeutic exposures of neratinib, there was no clinically relevant effect on the QTc interval. | ||
|PK= | |PK= | ||
*Neratinib exhibits a non-linear PK profile with less than dose proportional increase of AUC with the increasing daily dose over the range of 40 to 400 mg. | *Neratinib exhibits a non-linear PK profile with less than dose proportional increase of AUC with the increasing daily dose over the range of 40 to 400 mg. | ||
| Line 192: | Line 192: | ||
*The neratinib and major active metabolites M3, M6 and M7 peak concentrations are reached in the range of 2 to 8 hours after oral administration. | *The neratinib and major active metabolites M3, M6 and M7 peak concentrations are reached in the range of 2 to 8 hours after oral administration. | ||
''Effect of Food'' | ''Effect of Food'' | ||
*The food-effect assessment was conducted in healthy volunteers who received | *The food-effect assessment was conducted in healthy volunteers who received neratinib 240 mg under fasting conditions and with high fat food (approximately 55% fat, 31% carbohydrate, and 14% protein) or standard breakfast (approximately 50% carbohydrate, 35% fat, and 15% protein). A high fat meal increased neratinib C<sub>max</sub> and AUC<sub>inf</sub> by 1.7-fold (90% CI: 1.1- 2.7) and 2.2-fold (90% CI: 1.4- 3.5), respectively. A standard breakfast increased the C<sub>max</sub> and AUC<sub>inf</sub> by 1.2-fold (90% CI: 0.97- 1.42) and 1.1-fold (90% CI: 1.02- 1.24), respectively. | ||
=====Distribution===== | =====Distribution===== | ||
*In patients, following multiple doses of | *In patients, following multiple doses of neratinib, the mean (%CV) apparent volume of distribution at steady-state (V<sub>ss</sub>/F) was 6433 (19%) L. In vitro protein binding of neratinib in human plasma was greater than 99% and independent of concentration. Neratinib bound predominantly to human serum albumin and human alpha-1 acid glycoprotein. | ||
=====Elimination===== | =====Elimination===== | ||
*Following 7 days of daily 240 mg oral doses of | *Following 7 days of daily 240 mg oral doses of neratinib in healthy subjects, the mean (%CV) plasma half-life of neratinib, M3, M6, and M7 was 14.6 (38%), 21.6 (77%), 13.8 (50%) and 10.4 (33%) hours, respectively. The mean elimination half-life of neratinib ranged from 7 to 17 hours following a single oral dose in patients. Following multiple doses of neratinib at once-daily 240 mg in cancer patients, the mean (%CV) CL/F after first dose and at steady state (day 21) were 216 (34%) and 281 (40%) L/hour, respectively. | ||
''Metabolism'' | ''Metabolism'' | ||
*Neratinib is metabolized primarily in the liver by CYP3A4 and to a lesser extent by flavin-containing monooxygenase (FMO). | *Neratinib is metabolized primarily in the liver by CYP3A4 and to a lesser extent by flavin-containing monooxygenase (FMO). | ||
*After oral administration of | *After oral administration of neratinib, neratinib represents the most prominent component in plasma. At steady state after 240 mg daily oral doses of neratinib in a healthy subject study (n=25), the systemic exposures (AUC) of the active metabolites M3, M6, M7 and M11were 15%, 33%, 22% and 4% of the systemic neratinib exposure (AUC) respectively. | ||
''Excretion'' | ''Excretion'' | ||
*After oral administration of 200 mg (0.83 times of approved recommended dosage) radiolabeled neratinib oral formulation, fecal excretion accounted for approximately 97.1% and urinary excretion accounted for 1.13% of the total dose. Sixty-one percent of the excreted radioactivity was recovered within 96 hours and 98% was recovered after 10 days. | *After oral administration of 200 mg (0.83 times of approved recommended dosage) radiolabeled neratinib oral formulation, fecal excretion accounted for approximately 97.1% and urinary excretion accounted for 1.13% of the total dose. Sixty-one percent of the excreted radioactivity was recovered within 96 hours and 98% was recovered after 10 days. | ||
| Line 205: | Line 205: | ||
*Age, gender, race and renal function do not have a clinically significant effect on neratinib pharmacokinetics. | *Age, gender, race and renal function do not have a clinically significant effect on neratinib pharmacokinetics. | ||
''Patients with Hepatic Impairment'' | ''Patients with Hepatic Impairment'' | ||
*Neratinib is mainly metabolized in the liver. Single doses of 120 mg | *Neratinib is mainly metabolized in the liver. Single doses of 120 mg neratinib were evaluated in non-cancer patients with chronic hepatic impairment (n=6 each in Child Pugh Class A, B, and C) and in healthy subjects (n=9) with normal hepatic function. Neratinib exposures in the patients with Child Pugh Class A (mild impairment) and Child Pugh Class B (moderate impairment) were similar to that in normal healthy volunteers. Patients with severe hepatic impairment (Child Pugh Class C) had neratinib C<sub>max</sub> and AUC increased by 273% and 281%, respectively, as compared to the normal hepatic function controls. | ||
=====Drug Interaction Studies===== | =====Drug Interaction Studies===== | ||
*Gastric Acid Reducing Agents: | *Gastric Acid Reducing Agents: neratinib solubility decreases with increasing GI tract pH values. Drugs that alter the pH values of the GI tract may alter the solubility of neratinib and hence its absorption and systemic exposure. When multiple doses of lansoprazole (30 mg daily), a proton pump inhibitor, were co-administered with a single 240 mg oral doses of neratinib, the neratinib C<sub>max</sub> and AUC decreased by 71% and 65%, respectively. When a single oral dose of 240 mg neratinib was administered 2 hours following a daily dose of 300 mg ranitidine, an H-2 receptor antagonist, the neratinib C<sub>max</sub> and AUC were reduced by 57% and 48%, respectively. When a single oral dose of 240 mg neratinib was administered 2 hours prior to 150 mg ranitidine twice daily (administered in the morning and evening, approximately 12 hours apart), the neratinib C<sub>max</sub>and AUC were reduced by 44% and 32%, respectively. | ||
*Strong and Moderate CYP3A4 Inhibitors: Concomitant use of ketoconazole (400 mg once-daily for 5 days), a strong inhibitor of CYP3A4, with a single oral 240 mg | *Strong and Moderate CYP3A4 Inhibitors: Concomitant use of ketoconazole (400 mg once-daily for 5 days), a strong inhibitor of CYP3A4, with a single oral 240 mg neratinib dose in healthy subjects (n=24) increased neratinib C<sub>max</sub> by 321% and AUC by 481%. | ||
*The effect of moderate CYP3A4 inhibition has not been studied. Given neratinib is predominantly metabolized by the CYP3A4 pathway and had a significant exposure change with strong CYP3A4 inhibition, the potential impact on | *The effect of moderate CYP3A4 inhibition has not been studied. Given neratinib is predominantly metabolized by the CYP3A4 pathway and had a significant exposure change with strong CYP3A4 inhibition, the potential impact on neratinib safety from concomitant use with moderate CYP3A4 inhibitors warrants consideration. | ||
*Strong and Moderate CYP3A4 Inducers: Concomitant use of rifampin, a strong inducer of CYP3A4, with a single oral 240 mg | *Strong and Moderate CYP3A4 Inducers: Concomitant use of rifampin, a strong inducer of CYP3A4, with a single oral 240 mg neratinib dose in healthy subjects (n=24) reduced neratinib C<sub>max</sub> by 76% and AUC by 87%. The AUC of active metabolites M6 and M7 were also reduced by 37-49% when compared to neratinib administered alone. | ||
*The effect of moderate CYP3A4 induction has not been studied. Given neratinib is predominantly metabolized by the CYP3A4 pathway and had a significant exposure change with strong CYP3A4 induction, the potential impact on | *The effect of moderate CYP3A4 induction has not been studied. Given neratinib is predominantly metabolized by the CYP3A4 pathway and had a significant exposure change with strong CYP3A4 induction, the potential impact on neratinib efficacy from concomitant use with moderate CYP3A4 inducers warrants consideration. | ||
*Effect of | *Effect of neratinib on P-gp Transporters: Concomitant use of digoxin (a single 0.5 mg oral dose), a P-gp substrate, with multiple oral doses of neratinib 240 mg in healthy subjects (n=18) increased the mean digoxin C<sub>max</sub> by 54% and AUC by 32%. | ||
|nonClinToxic= | |nonClinToxic= | ||
=====Carcinogenesis, Mutagenesis, Impairment of Fertility===== | =====Carcinogenesis, Mutagenesis, Impairment of Fertility===== | ||
*A two-year carcinogenicity study was conducted in rats at oral neratinib doses of 1, 3, and 10 mg/kg/day. Neratinib was not carcinogenic in male and female rats at exposure levels > 25 times the AUC in patients receiving the maximum recommended dose of 240 mg/day. Neratinib was not carcinogenic in a 26-week study in Tg.rasH2 transgenic mice when administered daily by oral gavage at doses up to 50 mg/kg/day in males and 125 mg/kg/day in females. | *A two-year carcinogenicity study was conducted in rats at oral neratinib doses of 1, 3, and 10 mg/kg/day. Neratinib was not carcinogenic in male and female rats at exposure levels > 25 times the AUC in patients receiving the maximum recommended dose of 240 mg/day. Neratinib was not carcinogenic in a 26-week study in Tg.rasH2 transgenic mice when administered daily by oral gavage at doses up to 50 mg/kg/day in males and 125 mg/kg/day in females. | ||
*Neratinib was not mutagenic in an in vitro bacterial reverse mutation (AMES) assay or clastogenic in an in vitro human lymphocyte chromosomal aberration assay or an in vivo rat bone marrow micronucleus assay. | *Neratinib was not mutagenic in an in vitro bacterial reverse mutation (AMES) assay or clastogenic in an in vitro human lymphocyte chromosomal aberration assay or an in vivo rat bone marrow micronucleus assay. | ||
*In a fertility study in rats, neratinib administration up to 12 mg/kg/day (approximately 0.5 times the maximum recommended dose of 240 mg/day in patients on a mg/m 2 basis) caused no effects on mating or the ability of animals to become pregnant. In repeat-dose toxicity studies in dogs with oral administration of neratinib daily for up to 39 weeks, tubular hypoplasia of the testes was observed at ≥ 0.5 mg/kg/day. This finding was observed at AUCs that were approximately 0.4 times the AUC in patients at the maximum recommended dose of 240 mg. | *In a fertility study in rats, neratinib administration up to 12 mg/kg/day (approximately 0.5 times the maximum recommended dose of 240 mg/day in patients on a mg/m<sup>2</sup> basis) caused no effects on mating or the ability of animals to become pregnant. In repeat-dose toxicity studies in dogs with oral administration of neratinib daily for up to 39 weeks, tubular hypoplasia of the testes was observed at ≥ 0.5 mg/kg/day. This finding was observed at AUCs that were approximately 0.4 times the AUC in patients at the maximum recommended dose of 240 mg. | ||
|clinicalStudies= | |clinicalStudies= | ||
=====Extended Adjuvant Treatment in Breast Cancer===== | =====Extended Adjuvant Treatment in Breast Cancer===== | ||
*The safety and efficacy of | *The safety and efficacy of neratinib were investigated in the ExteNET trial (NCT00878709), a multicenter, randomized, double-blind, placebo-controlled study of neratinib after adjuvant treatment with trastuzumab in women with HER2-positive breast cancer. | ||
*A total of 2840 patients with early-stage HER2-positive breast cancer within two years of completing treatment with adjuvant trastuzumab was randomized to receive either | *A total of 2840 patients with early-stage HER2-positive breast cancer within two years of completing treatment with adjuvant trastuzumab was randomized to receive either neratinib (n=1420) or placebo (n=1420). Randomization was stratified by the following factors: hormone receptor status, nodal status (0, 1-3 vs 4 or more positive nodes) and whether trastuzumab was given sequentially versus concurrently with chemotherapy. Neratinib 240 mg or placebo was given orally once daily for one year. The major efficacy outcome measure was invasive disease-free survival (iDFS) defined as the time between the date of randomization to the first occurrence of invasive recurrence (local/regional, ipsilateral, or contralateral breast cancer), distant recurrence, or death from any cause, with 2 years and 28 days of follow-up. | ||
*Patient demographics and tumor characteristics were generally balanced between treatment arms. Patients had a median age of 52 years (range 23 to 83) and 12% of patients were 65 or older. The majority of patients were White (81%), and most patients (99.7%) had an ECOG performance status of 0 or 1. Fifty-seven percent (57%) had hormone receptor positive disease (defined as ER-positive and/or PgR-positive), 24% were node negative, 47% had one to three positive nodes and 30% had four or more positive nodes. Ten percent (10%) of patients had Stage I disease, 41% had Stage II disease and 31% had Stage III disease. The majority of patients (81%) were enrolled within one year of completion of trastuzumab treatment. Median time from the last adjuvant trastuzumab treatment to randomization was 4.4 months in the | *Patient demographics and tumor characteristics were generally balanced between treatment arms. Patients had a median age of 52 years (range 23 to 83) and 12% of patients were 65 or older. The majority of patients were White (81%), and most patients (99.7%) had an ECOG performance status of 0 or 1. Fifty-seven percent (57%) had hormone receptor positive disease (defined as ER-positive and/or PgR-positive), 24% were node negative, 47% had one to three positive nodes and 30% had four or more positive nodes. Ten percent (10%) of patients had Stage I disease, 41% had Stage II disease and 31% had Stage III disease. The majority of patients (81%) were enrolled within one year of completion of trastuzumab treatment. Median time from the last adjuvant trastuzumab treatment to randomization was 4.4 months in the neratinib arm vs. 4.6 months in the placebo arm. Median duration of treatment was 11.6 months in the neratinib arm vs. 11.8 months in the placebo arm. | ||
*The efficacy results from the ExteNET trial are summarized in TABLE 8 and FIGURE 1. | *The efficacy results from the ExteNET trial are summarized in TABLE 8 and FIGURE 1. | ||
[[image:neratinibtrial1.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | [[image:neratinibtrial1.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | ||
| Line 229: | Line 229: | ||
*Approximately 75% of patients were re-consented for extended follow-up beyond 24 months. Observations with missing data were censored at the last date of assessment. This exploratory analysis suggests that the iDFS results at 5 years are consistent with the 2-year iDFS results observed in ExteNET. At the time of the iDFS analysis, 2% of patients had died, and Overall Survival data were immature. | *Approximately 75% of patients were re-consented for extended follow-up beyond 24 months. Observations with missing data were censored at the last date of assessment. This exploratory analysis suggests that the iDFS results at 5 years are consistent with the 2-year iDFS results observed in ExteNET. At the time of the iDFS analysis, 2% of patients had died, and Overall Survival data were immature. | ||
|howSupplied= | |howSupplied= | ||
* | *Neratinib 40 mg film-coated tablets are red, oval shaped and debossed with ‘W104’ on one side and plain on the other side. | ||
* | *Neratinib is available in: | ||
:*Bottles of 180 tablets: NDC 70437-240-18 | :*Bottles of 180 tablets: NDC 70437-240-18 | ||
:*Bottles of 126 tablets: NDC 70437-240-26 | :*Bottles of 126 tablets: NDC 70437-240-26 | ||
| Line 243: | Line 243: | ||
*Advise the patient to read the FDA-approved patient labeling | *Advise the patient to read the FDA-approved patient labeling | ||
=====Diarrhea===== | =====Diarrhea===== | ||
*Inform patients that | *Inform patients that neratinib has been associated with diarrhea which may be severe in some cases. | ||
*Instruct patients to maintain 1-2 bowel movements per day and on how to use anti-diarrheal treatment regimens. | *Instruct patients to maintain 1-2 bowel movements per day and on how to use anti-diarrheal treatment regimens. | ||
*Advise patients to inform their healthcare provider immediately if severe (≥Grade 3) diarrhea or diarrhea associated with weakness, dizziness, or fever occurs during treatment with | *Advise patients to inform their healthcare provider immediately if severe (≥Grade 3) diarrhea or diarrhea associated with weakness, dizziness, or fever occurs during treatment with neratinib. | ||
=====Hepatotoxicity===== | =====Hepatotoxicity===== | ||
*Inform patients that | *Inform patients that neratinib has been associated with hepatotoxicity which may be severe in some cases. | ||
Inform patients that they should report signs and symptoms of liver dysfunction to their healthcare provider immediately. | Inform patients that they should report signs and symptoms of liver dysfunction to their healthcare provider immediately. | ||
=====Embryo-Fetal Toxicity===== | =====Embryo-Fetal Toxicity===== | ||
*Advise females to inform their healthcare provider if they are pregnant or become pregnant. Inform female patients of the risk to a fetus and potential loss of the pregnancy. | *Advise females to inform their healthcare provider if they are pregnant or become pregnant. Inform female patients of the risk to a fetus and potential loss of the pregnancy. | ||
*Advise females of reproductive potential to use effective contraception during treatment and for 1 month after receiving the last dose of | *Advise females of reproductive potential to use effective contraception during treatment and for 1 month after receiving the last dose of neratinib. | ||
*Advise lactating women not to breastfeed during treatment with | *Advise lactating women not to breastfeed during treatment with neratinib and for at least 1 month after the last dose. | ||
=====Drug Interactions===== | =====Drug Interactions===== | ||
* | *Neratinib may interact with many drugs; therefore, advise patients to report to their healthcare provider the use of any other prescription or nonprescription medication or herbal products. | ||
* | *Neratinib may interact with gastric acid reducing agents. Advise patients to avoid concomitant use of proton pump inhibitors. When patients require gastric acid reducing agents, use an H 2-receptor antagonist or antacid. Advise patients to separate the dosing of neratinib by 3 hours after antacid medicine, and to take neratinib at least 2 hours before or 10 hours after a H 2-receptor antagonist. | ||
* | *Neratinib may interact with grapefruit. Advise patients to avoid taking neratinib with grapefruit products. | ||
=====Dosing and Administration===== | =====Dosing and Administration===== | ||
*Instruct patients to take | *Instruct patients to take neratinib with food at approximately the same time each day consecutively for one year. | ||
*If a patient misses a dose, instruct the patient not to replace the missed dose, and to resume | *If a patient misses a dose, instruct the patient not to replace the missed dose, and to resume neratinib with the next scheduled daily dose. | ||
[[image:neratinibpatientinsert.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | [[image:neratinibpatientinsert.png|none|thumb|400px|This image is provided by the National Library of Medicine.]] | ||
|nlmPatientInfo= | |nlmPatientInfo= | ||
Latest revision as of 14:57, 20 July 2018
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sonya Gelfand
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Neratinib is a kinase inhibitor that is FDA approved for the extended adjuvant treatment of adult patients with early stage HER2-overexpressed/amplified breast cancer, to follow adjuvant trastuzumab-based therapy. Common adverse reactions include diarrhea, nausea, abdominal pain, fatigue, vomiting, rash, stomatitis, decreased appetite, muscle spasms, dyspepsia, AST or ALT increase, nail disorder, dry skin, abdominal distention, weight decreased and urinary tract infection.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
- Neratinib is indicated for the extended adjuvant treatment of adult patients with early stage HER2-overexpressed/amplified breast cancer, to follow adjuvant trastuzumab based therapy.
Antidiarrheal Prophylaxis
- Antidiarrheal prophylaxis is recommended during the first 2 cycles (56 days) of treatment and should be initiated with the first dose of neratinib.
- Instruct patients to take loperamide as directed in TABLE 1, titrating to 1-2 bowel movements per day.
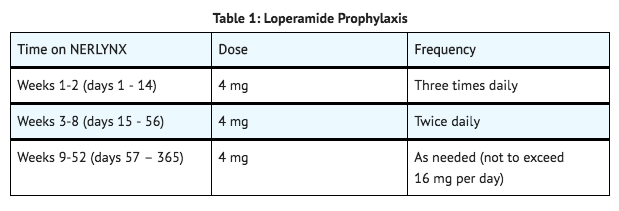
- Additional antidiarrheal agents may be required to manage diarrhea in patients with loperamide-refractory diarrhea. Neratinib dose interruptions and dose reductions may also be required to manage diarrhea.
Recommended Dose and Schedule
- The recommended dose of neratinib is 240 mg (six tablets) given orally once daily with food, continuously for one year.
- Instruct patients to take neratinib at approximately the same time every day. Neratinib tablets should be swallowed whole (tablets should not be chewed, crushed, or split prior to swallowing).
- If a patient misses a dose, do not replace missed dose, and instruct the patient to resume neratinib with the next scheduled daily dose.
Dose Modifications
Dose Modifications for Adverse Reactions
- Neratinib dose modification is recommended based on individual safety and tolerability. Management of some adverse reactions may require dose interruption and/or dose reduction as shown in TABLE 2 to TABLE 5. Discontinue neratinib for patients who fail to recover to Grade 0-1 from treatment-related toxicity, for toxicities that result in a treatment delay > 3 weeks, or for patients that are unable to tolerate 120 mg daily. Additional clinical situations may result in dose adjustments as clinically indicated (e.g. intolerable toxicities, persistent Grade 2 adverse reactions, etc.).
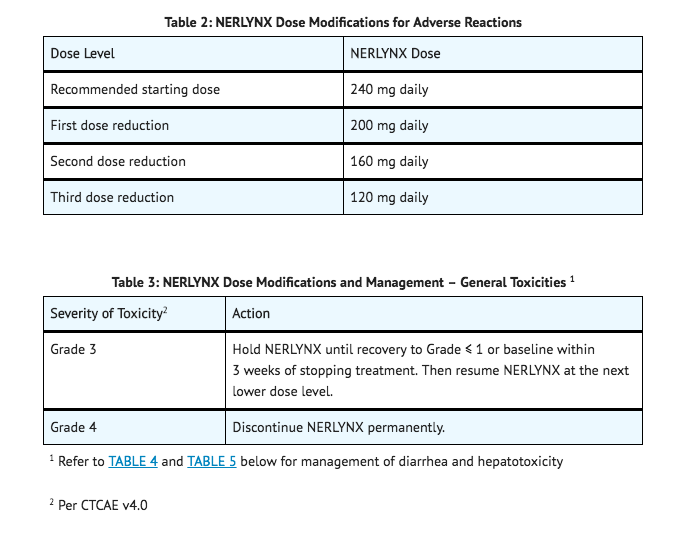
Dose Modifications for Diarrhea
- Diarrhea management requires the correct use of antidiarrheal medication, dietary changes, and appropriate dose modifications of neratinib. Guidelines for adjusting doses of neratinib in the setting of diarrhea are shown in TABLE 4.
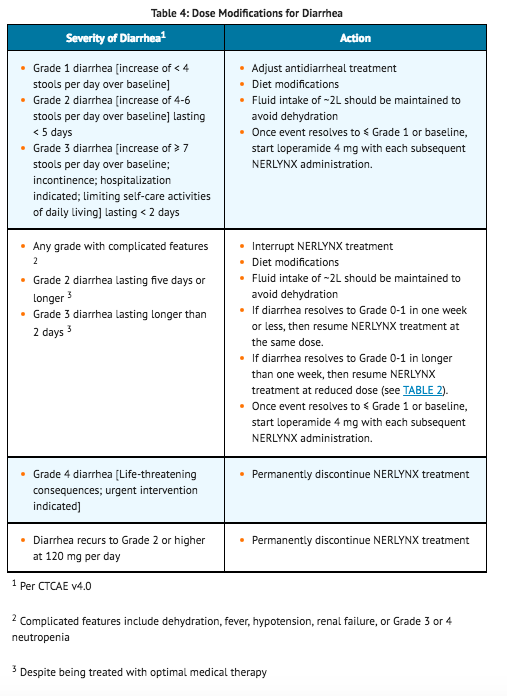
Dose Modifications for Hepatic Impairment
- Reduce the neratinib starting dose to 80 mg in patients with severe hepatic impairment (Child Pugh C). No dose modifications are recommended for patients with mild to moderate hepatic impairment (Child Pugh A or B).
Dose Modifications for Hepatotoxicity
- Guidelines for dose adjustment of neratinib in the event of liver toxicity are shown in TABLE 5. Patients who experience ≥ Grade 3 diarrhea requiring IV fluid treatment or any signs or symptoms of hepatotoxicity, such as worsening of fatigue, nausea, vomiting, right upper quadrant pain or tenderness, fever, rash, or eosinophilia, should be evaluated for changes in liver function tests. Fractionated bilirubin and prothrombin time should also be collected during hepatotoxicity evaluation.
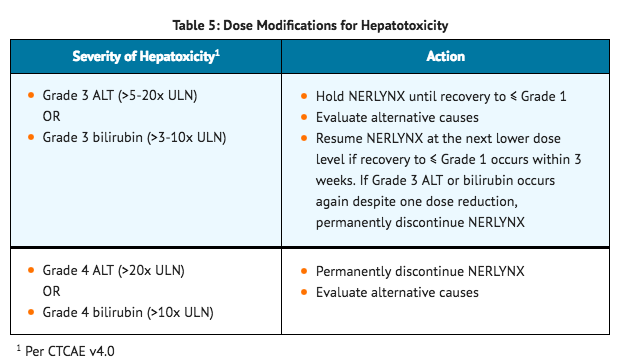
Concomitant Use with Gastric Acid Reducing Agents
- Proton pump inhibitors (PPI): Avoid concomitant use with neratinib.
- H2-receptor antagonists: Take neratinib at least 2 hours before the next dose of the H 2-receptor antagonist or 10 hours after the H2-receptor antagonist.
- Antacids: Separate dosing of neratinib by 3 hours after antacids.
Dosage Forms and Strengths
- Tablets: 40 mg neratinib (equivalent to 48.31 mg of neratinib maleate).
- Film-coated, red, oval shaped and debossed with ‘W104’ on one side and plain on the other side.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding neratinib Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding neratinib Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Neratinib FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding neratinib Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding neratinib Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
- None.
Warnings
Diarrhea
- Severe diarrhea and sequelae, such as dehydration, hypotension, and renal failure, have been reported during treatment with neratinib. Diarrhea was reported in 95% of neratinib-treated patients in ExteNET, a randomized placebo controlled trial. In the neratinib arm, Grade 3 diarrhea occurred in 40% and Grade 4 diarrhea occurred in 0.1% of patients. The majority of patients (93%) had diarrhea in the first month of treatment, the median time to first onset of Grade ≥ 3 diarrhea was 8 days (range, 1-350), and the median cumulative duration of Grade ≥ 3 diarrhea was 5 days (range, 1-139).
- Antidiarrheal prophylaxis with loperamide has been shown to lower the incidence and severity of diarrhea. Instruct patients to initiate antidiarrheal prophylaxis with loperamide along with the first dose of neratinib and continue during the first two cycles (56 days) of treatment.
- Monitor patients for diarrhea and treat with additional antidiarrheals as needed. When severe diarrhea with dehydration occurs, administer fluid and electrolytes as needed, interrupt neratinib, and reduce subsequent doses. Perform stool cultures as clinically indicated to exclude infectious causes of Grade 3 or 4 diarrhea or diarrhea of any grade with complicating features (dehydration, fever, neutropenia).
Hepatotoxicity
- Neratinib has been associated with hepatotoxicity characterized by increased liver enzymes. In ExteNET, 9.7% of patients experienced an alanine aminotransferase (ALT) increase ≥ 2 x ULN, 5.1% of patients experienced an aspartate aminotransferase (AST) increase ≥ 2 x ULN, and 1.7% of patients experienced an AST or ALT elevation > 5 x ULN (≥ Grade 3). Hepatotoxicity or increases in liver transaminases led to drug discontinuation in 1.7% of neratinib-treated patients.
- Total bilirubin, AST, ALT, and alkaline phosphatase should be measured prior to starting treatment with neratinib monthly for the first 3 months of treatment, then every 3 months while on treatment and as clinically indicated. These tests should also be performed in patients experiencing Grade 3 diarrhea or any signs or symptoms of hepatotoxicity, such as worsening of fatigue, nausea, vomiting, right upper quadrant tenderness, fever, rash, or eosinophilia.
Embryo-Fetal Toxicity
- Based on findings from animal studies and its mechanism of action, neratinib can cause fetal harm when administered to a pregnant woman. In animal reproduction studies, administration of neratinib to pregnant rabbits during organogenesis caused abortions, embryo-fetal death and fetal abnormalities in rabbits at maternal AUCs approximately 0.2 times the AUC in patients receiving the recommended dose. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment and for at least 1 month after the last dose.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
ExteNET
- The data described below reflect exposure of neratinib as a single agent in ExteNET, a multicenter, randomized, double-blind, placebo-controlled study of neratinib within 2 years after completion of adjuvant treatment with trastuzumab-based therapy in women with HER2-positive early-stage breast cancer. Patients who received neratinib in this trial were not required to receive any prophylaxis with antidiarrheal agents to prevent the neratinib-related diarrhea. The median duration of treatment was 11.6 months in the neratinib arm and 11.8 months in the placebo arm. The median age was 52 years (60% were ≥ 50 years old, 12% were ≥ 65 years old); 81% were Caucasian, 3% Black or African American, 14% Asian and 3% other. A total of 1408 patients were treated with neratinib.
- Neratinib dose reduction due to an adverse reaction of any grade occurred in 31.2% of patients receiving neratinib compared to 2.6% of patients receiving placebo. Permanent discontinuation due to any adverse reaction was reported in 27.6% of neratinib-treated patients. The most common adverse reaction leading to discontinuation was diarrhea, accounting for 16.8% of neratinib-treated patients.
- The most common adverse reactions (>5%) were diarrhea, nausea, abdominal pain, fatigue, vomiting, rash, stomatitis, decreased appetite, muscle spasms, dyspepsia, AST or ALT increase, nail disorder, dry skin, abdominal distention, weight decreased and urinary tract infection. The most frequently reported Grade 3 or 4 adverse reactions were diarrhea, vomiting, nausea, and abdominal pain.
- Serious adverse reactions in the neratinib arm included diarrhea (1.6%), vomiting (0.9%), dehydration (0.6%), cellulitis (0.4%), renal failure (0.4%), erysipelas (0.4%), alanine aminotransferase increased (0.3%), aspartate aminotransferase increased (0.3%), nausea (0.3%), fatigue (0.2%), and abdominal pain (0.2%).
- TABLE 6 summarizes the adverse reactions in ExteNET.
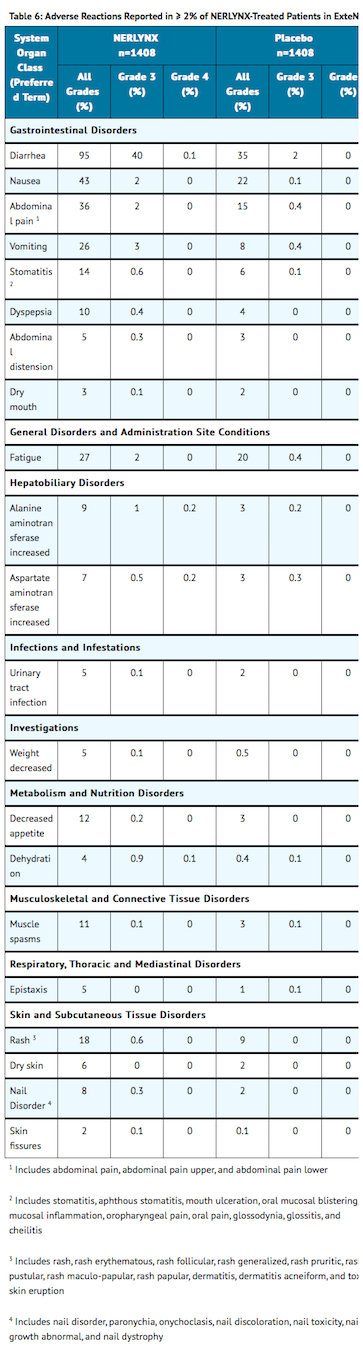
Postmarketing Experience
There is limited information regarding Neratinib Postmarketing Experience in the drug label.
Drug Interactions
- Effect of Other Drugs on Neratinib
- Effect of Neratinib on Other Drugs
Effect of Other Drugs on Neratinib
- TABLE 7 includes drug interactions that affect the pharmacokinetics of neratinib.
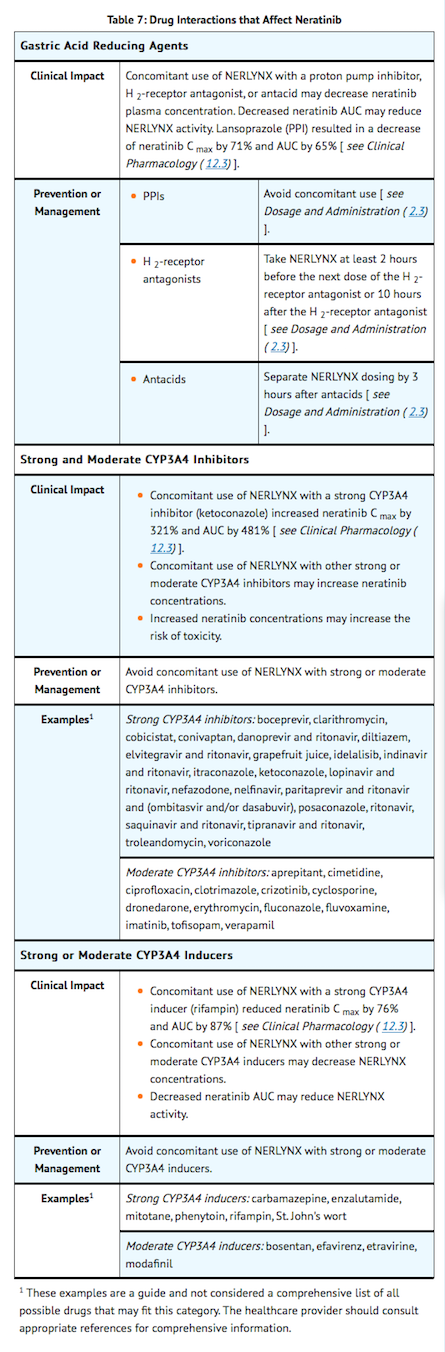
Effect of Neratinib on Other Drugs
P-glycoprotein (P-gp) Substrates
- Concomitant use of neratinib with digoxin, a P-gp substrate, increased digoxin concentrations. Increased concentrations of digoxin may lead to increased risk of adverse reactions including cardiac toxicity. Refer to the digoxin prescribing information for dosage adjustment recommendations due to drug interactions. neratinib may inhibit the transport of other P-gp substrates (e.g., dabigatran, fexofenadine).
Use in Specific Populations
Pregnancy
Risk Summary
- Based on findings from animal studies and the mechanism of action, neratinib can cause fetal harm when administered to a pregnant woman.
- There are no available data in pregnant women to inform the drug-associated risk. In animal reproduction studies, administration of neratinib to pregnant rabbits during organogenesis resulted in abortions, embryo-fetal death and fetal abnormalities in rabbits at maternal exposures (AUC) approximately 0.2 times exposures in patients at the recommended dose. Advise pregnant women of the potential risk to a fetus.
- The background risk of major birth defects and miscarriage for the indicated population is unknown. However, the background risk of major birth defects is 2-4% and of miscarriage is 15-20% of clinically recognized pregnancies in the U.S. general population.
Data (Animal)
- In a fertility and early embryonic development study in female rats, neratinib was administered orally for 15 days before mating to Day 7 of pregnancy, which did not cause embryonic toxicity at doses up to 12 mg/kg/day in the presence of maternal toxicity. A dose of 12 mg/kg/day in rats is approximately 0.5 times the maximum recommended dose of 240 mg/day in patients on a mg/m2 basis.
- In an embryo-fetal development study in rats, pregnant animals received oral doses of neratinib up to 15 mg/kg/day during the period of organogenesis. No effects on embryo-fetal development or survival were observed. Maternal toxicity was evident at 15 mg/kg/day (approximately 0.6 times the AUC in patients receiving the maximum recommended dose of 240 mg/day).
- In an embryo-fetal development study in rabbits, pregnant animals received oral doses of neratinib up to 9 mg/kg/day during the period of organogenesis. Administration of neratinib at doses ≥ 6 mg/kg/day resulted in maternal toxicity, abortions and embryo-fetal death (increased resorptions). Neratinib administration resulted in increased incidence of fetal gross external (domed head), soft tissue (dilation of the brain ventricles and ventricular septal defect), and skeletal (misshapen anterior fontanelles and enlarged anterior and/or posterior fontanelles) abnormalities at ≥ 3 mg/kg/day. The AUC(0-t) at 6 mg/kg/day and 9 mg/kg/day in rabbits were approximately 0.5 and 0.8 times, respectively, the AUCs in patients receiving the maximum recommended dose of 240 mg/day.
- In a peri and postnatal development study in rats, oral administration of neratinib from gestation day 7 until lactation day 20 resulted in maternal toxicity at ≥ 10 mg/kg/day (approximately 0.4 times the maximum recommended dose of 240 mg/day in patients on a mg/m2 basis) including decreased body weights, body weight gains, and food consumption. Effects on long-term memory were observed in male offspring at maternal doses ≥ 5 mg/kg/day (approximately 0.2 times the maximum recommended dose of 240 mg/day in patients on a mg/m2 basis).
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Neratinib in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Neratinib during labor and delivery.
Nursing Mothers
Risk Summary
- No data are available regarding the presence of neratinib or its metabolites in human milk or its effects on the breastfed infant or on milk production. Because of the potential for serious adverse reactions in breastfed infants from neratinib, advise lactating women not to breastfeed while taking neratinib and for at least 1 month after the last dose.
Pediatric Use
- The safety and efficacy of neratinib in pediatric patients has not been established.
Geriatic Use
- In the ExteNET trial, the mean age was 52 years in the neratinib arm; 1236 patients were < 65 years, 172 patients were ≥ 65 years, of whom 25 patients were 75 years or older.
- There was a higher frequency of treatment discontinuations due to adverse reactions in the ≥ 65 years age group than in the < 65 years age group; in the neratinib arm, the percentages were 44.8% compared with 25.2%, respectively, and in the placebo arm 6.4% and 5.3%, respectively.
- The incidence of serious adverse reactions in the neratinib arm vs. placebo arm was 7.0% vs. 5.7% (< 65 years-old) and 9.9% vs. 8.1% (≥ 65 years-old). The serious adverse reactions most frequently reported in the ≥ 65 years-old group were vomiting (2.3%), diarrhea (1.7%), renal failure (1.7%), and dehydration (1.2%).
Gender
There is no FDA guidance on the use of Neratinib with respect to specific gender populations.
Race
There is no FDA guidance on the use of Neratinib with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Neratinib in patients with renal impairment.
Hepatic Impairment
- No dose modifications are recommended for patients with mild to moderate hepatic impairment (Child Pugh A or B). Patients with severe, pre-existing hepatic impairment (Child Pugh Class C) experienced a reduction in neratinib clearance and an increase in Cmax and AUC. Reduce the neratinib dosage for patients with severe hepatic impairment.
Females of Reproductive Potential and Males
Pregnancy
- Based on animal studies, neratinib can cause fetal harm when administered to a pregnant woman. Females of reproductive potential should have a pregnancy test prior to starting treatment with neratinib.
Contraception
Females
- Based on animal studies, neratinib can cause fetal harm when administered to a pregnant woman. Advise females of reproductive potential to use effective contraception during treatment with neratinib and for at least 1 month after the last dose.
Males
- Based on findings in animal reproduction studies, advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 3 months after the last dose of neratinib.
Immunocompromised Patients
There is no FDA guidance one the use of Neratinib in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
- Disease response or stabilization may indicate efficacy.
- FTL3 mutation: Prior to initiation, with an FDA-approved companion diagnostic test available at http://www.fda/gov/CompanionDiagnostics.
- Pregnancy status (women of reproductive potential): Within 7 days prior to initiation of therapy.
- CBC: At least weekly for the first 4 weeks, every other week for the next 8 weeks, and monthly thereafter during therapy; include a differential.
- Toxicities, including nausea, vomiting, and other non-hematologic toxicities: At least weekly for the first 4 weeks, every other week for the next 8 weeks, and monthly thereafter during therapy.
- Pulmonary symptoms of interstitial lung disease or pneumonitis.
IV Compatibility
There is limited information regarding the compatibility of Neratinib and IV administrations.
Overdosage
- There is no specific antidote, and the benefit of hemodialysis in the treatment of neratinib overdose is unknown. In the event of an overdose, administration should be withheld and general supportive measures undertaken.
- In the clinical trial setting, a limited number of patients reported overdose. The adverse reactions experienced by these patients were diarrhea, nausea, vomiting, and dehydration. The frequency and severity of gastrointestinal disorders (diarrhea, abdominal pain, nausea and vomiting) appear to be dose related.
Pharmacology
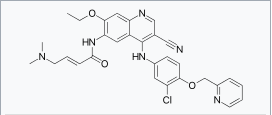
| |
Neratinib
| |
| Systematic (IUPAC) name | |
| (2E)-N-[4-[[3-Chloro-4-[(pyridin-2-yl)methoxy]phenyl]amino]-3-cyano-7-ethoxyquinolin-6-yl]-4-(dimethylamino)but-2-enamide | |
| Identifiers | |
| CAS number | |
| ATC code | none |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 557.04 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | Oral |
Mechanism of Action
- Neratinib is a kinase inhibitor that irreversibly binds to Epidermal Growth Factor Receptor (EGFR), Human Epidermal Growth Factor Receptor 2 (HER2), and HER4. In vitro, neratinib reduces EGFR and HER2 autophosphorylation, downstream MAPK and AKT signaling pathways, and showed antitumor activity in EGFR and/or HER2 expressing carcinoma cell lines. Neratinib human metabolites M3, M6, M7 and M11 inhibited the activity of EGFR, HER2 and HER4 in vitro. In vivo, oral administration of neratinib inhibited tumor growth in mouse xenograft models with tumor cell lines expressing HER2 and EGFR.
Structure
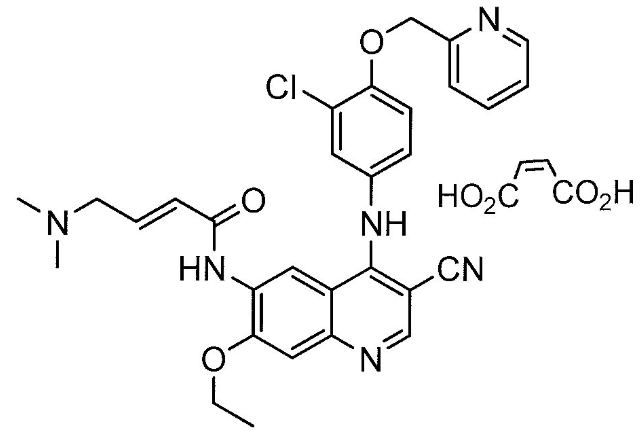
Pharmacodynamics
Cardiac Electrophysiology
- The effect of neratinib on the QTc interval was evaluated in a randomized, placebo and positive controlled, double-blind, single-dose, crossover study in 60 healthy subjects. At 2.4-fold the therapeutic exposures of neratinib, there was no clinically relevant effect on the QTc interval.
Pharmacokinetics
- Neratinib exhibits a non-linear PK profile with less than dose proportional increase of AUC with the increasing daily dose over the range of 40 to 400 mg.
Absorption
- The neratinib and major active metabolites M3, M6 and M7 peak concentrations are reached in the range of 2 to 8 hours after oral administration.
Effect of Food
- The food-effect assessment was conducted in healthy volunteers who received neratinib 240 mg under fasting conditions and with high fat food (approximately 55% fat, 31% carbohydrate, and 14% protein) or standard breakfast (approximately 50% carbohydrate, 35% fat, and 15% protein). A high fat meal increased neratinib Cmax and AUCinf by 1.7-fold (90% CI: 1.1- 2.7) and 2.2-fold (90% CI: 1.4- 3.5), respectively. A standard breakfast increased the Cmax and AUCinf by 1.2-fold (90% CI: 0.97- 1.42) and 1.1-fold (90% CI: 1.02- 1.24), respectively.
Distribution
- In patients, following multiple doses of neratinib, the mean (%CV) apparent volume of distribution at steady-state (Vss/F) was 6433 (19%) L. In vitro protein binding of neratinib in human plasma was greater than 99% and independent of concentration. Neratinib bound predominantly to human serum albumin and human alpha-1 acid glycoprotein.
Elimination
- Following 7 days of daily 240 mg oral doses of neratinib in healthy subjects, the mean (%CV) plasma half-life of neratinib, M3, M6, and M7 was 14.6 (38%), 21.6 (77%), 13.8 (50%) and 10.4 (33%) hours, respectively. The mean elimination half-life of neratinib ranged from 7 to 17 hours following a single oral dose in patients. Following multiple doses of neratinib at once-daily 240 mg in cancer patients, the mean (%CV) CL/F after first dose and at steady state (day 21) were 216 (34%) and 281 (40%) L/hour, respectively.
Metabolism
- Neratinib is metabolized primarily in the liver by CYP3A4 and to a lesser extent by flavin-containing monooxygenase (FMO).
- After oral administration of neratinib, neratinib represents the most prominent component in plasma. At steady state after 240 mg daily oral doses of neratinib in a healthy subject study (n=25), the systemic exposures (AUC) of the active metabolites M3, M6, M7 and M11were 15%, 33%, 22% and 4% of the systemic neratinib exposure (AUC) respectively.
Excretion
- After oral administration of 200 mg (0.83 times of approved recommended dosage) radiolabeled neratinib oral formulation, fecal excretion accounted for approximately 97.1% and urinary excretion accounted for 1.13% of the total dose. Sixty-one percent of the excreted radioactivity was recovered within 96 hours and 98% was recovered after 10 days.
Specific Populations
- Age, gender, race and renal function do not have a clinically significant effect on neratinib pharmacokinetics.
Patients with Hepatic Impairment
- Neratinib is mainly metabolized in the liver. Single doses of 120 mg neratinib were evaluated in non-cancer patients with chronic hepatic impairment (n=6 each in Child Pugh Class A, B, and C) and in healthy subjects (n=9) with normal hepatic function. Neratinib exposures in the patients with Child Pugh Class A (mild impairment) and Child Pugh Class B (moderate impairment) were similar to that in normal healthy volunteers. Patients with severe hepatic impairment (Child Pugh Class C) had neratinib Cmax and AUC increased by 273% and 281%, respectively, as compared to the normal hepatic function controls.
Drug Interaction Studies
- Gastric Acid Reducing Agents: neratinib solubility decreases with increasing GI tract pH values. Drugs that alter the pH values of the GI tract may alter the solubility of neratinib and hence its absorption and systemic exposure. When multiple doses of lansoprazole (30 mg daily), a proton pump inhibitor, were co-administered with a single 240 mg oral doses of neratinib, the neratinib Cmax and AUC decreased by 71% and 65%, respectively. When a single oral dose of 240 mg neratinib was administered 2 hours following a daily dose of 300 mg ranitidine, an H-2 receptor antagonist, the neratinib Cmax and AUC were reduced by 57% and 48%, respectively. When a single oral dose of 240 mg neratinib was administered 2 hours prior to 150 mg ranitidine twice daily (administered in the morning and evening, approximately 12 hours apart), the neratinib Cmaxand AUC were reduced by 44% and 32%, respectively.
- Strong and Moderate CYP3A4 Inhibitors: Concomitant use of ketoconazole (400 mg once-daily for 5 days), a strong inhibitor of CYP3A4, with a single oral 240 mg neratinib dose in healthy subjects (n=24) increased neratinib Cmax by 321% and AUC by 481%.
- The effect of moderate CYP3A4 inhibition has not been studied. Given neratinib is predominantly metabolized by the CYP3A4 pathway and had a significant exposure change with strong CYP3A4 inhibition, the potential impact on neratinib safety from concomitant use with moderate CYP3A4 inhibitors warrants consideration.
- Strong and Moderate CYP3A4 Inducers: Concomitant use of rifampin, a strong inducer of CYP3A4, with a single oral 240 mg neratinib dose in healthy subjects (n=24) reduced neratinib Cmax by 76% and AUC by 87%. The AUC of active metabolites M6 and M7 were also reduced by 37-49% when compared to neratinib administered alone.
- The effect of moderate CYP3A4 induction has not been studied. Given neratinib is predominantly metabolized by the CYP3A4 pathway and had a significant exposure change with strong CYP3A4 induction, the potential impact on neratinib efficacy from concomitant use with moderate CYP3A4 inducers warrants consideration.
- Effect of neratinib on P-gp Transporters: Concomitant use of digoxin (a single 0.5 mg oral dose), a P-gp substrate, with multiple oral doses of neratinib 240 mg in healthy subjects (n=18) increased the mean digoxin Cmax by 54% and AUC by 32%.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- A two-year carcinogenicity study was conducted in rats at oral neratinib doses of 1, 3, and 10 mg/kg/day. Neratinib was not carcinogenic in male and female rats at exposure levels > 25 times the AUC in patients receiving the maximum recommended dose of 240 mg/day. Neratinib was not carcinogenic in a 26-week study in Tg.rasH2 transgenic mice when administered daily by oral gavage at doses up to 50 mg/kg/day in males and 125 mg/kg/day in females.
- Neratinib was not mutagenic in an in vitro bacterial reverse mutation (AMES) assay or clastogenic in an in vitro human lymphocyte chromosomal aberration assay or an in vivo rat bone marrow micronucleus assay.
- In a fertility study in rats, neratinib administration up to 12 mg/kg/day (approximately 0.5 times the maximum recommended dose of 240 mg/day in patients on a mg/m2 basis) caused no effects on mating or the ability of animals to become pregnant. In repeat-dose toxicity studies in dogs with oral administration of neratinib daily for up to 39 weeks, tubular hypoplasia of the testes was observed at ≥ 0.5 mg/kg/day. This finding was observed at AUCs that were approximately 0.4 times the AUC in patients at the maximum recommended dose of 240 mg.
Clinical Studies
Extended Adjuvant Treatment in Breast Cancer
- The safety and efficacy of neratinib were investigated in the ExteNET trial (NCT00878709), a multicenter, randomized, double-blind, placebo-controlled study of neratinib after adjuvant treatment with trastuzumab in women with HER2-positive breast cancer.
- A total of 2840 patients with early-stage HER2-positive breast cancer within two years of completing treatment with adjuvant trastuzumab was randomized to receive either neratinib (n=1420) or placebo (n=1420). Randomization was stratified by the following factors: hormone receptor status, nodal status (0, 1-3 vs 4 or more positive nodes) and whether trastuzumab was given sequentially versus concurrently with chemotherapy. Neratinib 240 mg or placebo was given orally once daily for one year. The major efficacy outcome measure was invasive disease-free survival (iDFS) defined as the time between the date of randomization to the first occurrence of invasive recurrence (local/regional, ipsilateral, or contralateral breast cancer), distant recurrence, or death from any cause, with 2 years and 28 days of follow-up.
- Patient demographics and tumor characteristics were generally balanced between treatment arms. Patients had a median age of 52 years (range 23 to 83) and 12% of patients were 65 or older. The majority of patients were White (81%), and most patients (99.7%) had an ECOG performance status of 0 or 1. Fifty-seven percent (57%) had hormone receptor positive disease (defined as ER-positive and/or PgR-positive), 24% were node negative, 47% had one to three positive nodes and 30% had four or more positive nodes. Ten percent (10%) of patients had Stage I disease, 41% had Stage II disease and 31% had Stage III disease. The majority of patients (81%) were enrolled within one year of completion of trastuzumab treatment. Median time from the last adjuvant trastuzumab treatment to randomization was 4.4 months in the neratinib arm vs. 4.6 months in the placebo arm. Median duration of treatment was 11.6 months in the neratinib arm vs. 11.8 months in the placebo arm.
- The efficacy results from the ExteNET trial are summarized in TABLE 8 and FIGURE 1.
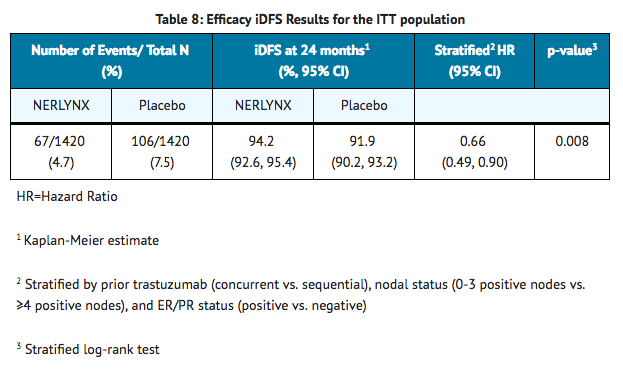
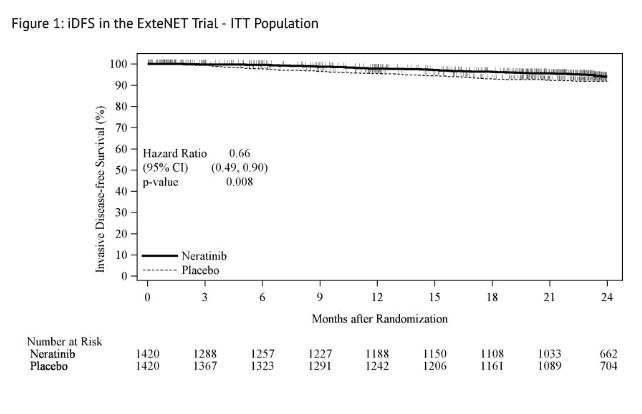
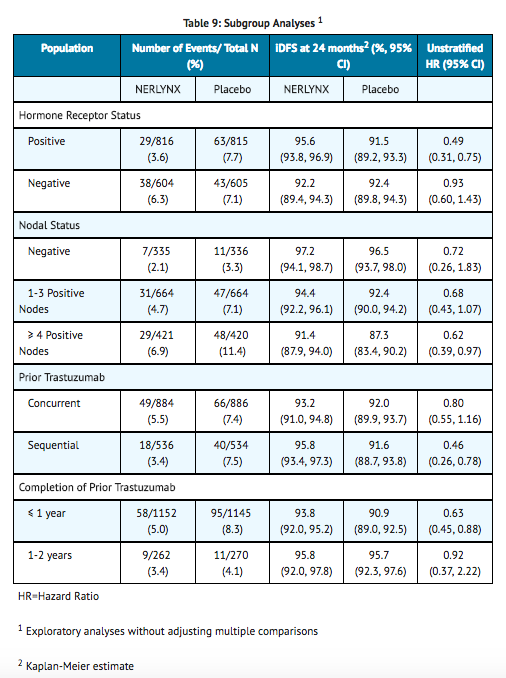
- Approximately 75% of patients were re-consented for extended follow-up beyond 24 months. Observations with missing data were censored at the last date of assessment. This exploratory analysis suggests that the iDFS results at 5 years are consistent with the 2-year iDFS results observed in ExteNET. At the time of the iDFS analysis, 2% of patients had died, and Overall Survival data were immature.
How Supplied
- Neratinib 40 mg film-coated tablets are red, oval shaped and debossed with ‘W104’ on one side and plain on the other side.
- Neratinib is available in:
- Bottles of 180 tablets: NDC 70437-240-18
- Bottles of 126 tablets: NDC 70437-240-26
Storage
- Store at controlled room temperature, 20°C to 25°C (68°F to 77°F); excursions permitted to 15-30°C (59–86°F).
Images
Drug Images
{{#ask: Page Name::Neratinib |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel




{{#ask: Label Page::Neratinib |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
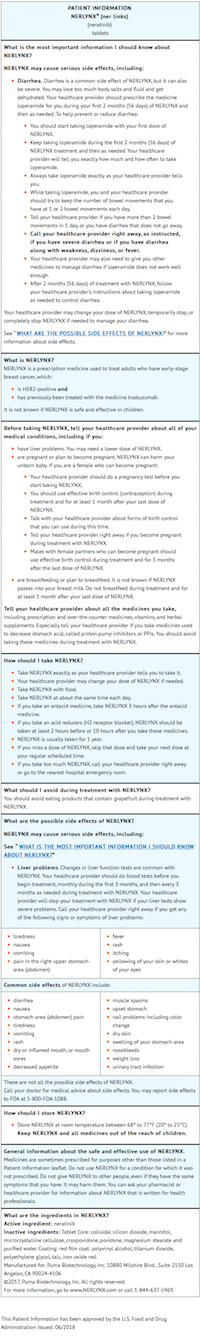
- Advise the patient to read the FDA-approved patient labeling
Diarrhea
- Inform patients that neratinib has been associated with diarrhea which may be severe in some cases.
- Instruct patients to maintain 1-2 bowel movements per day and on how to use anti-diarrheal treatment regimens.
- Advise patients to inform their healthcare provider immediately if severe (≥Grade 3) diarrhea or diarrhea associated with weakness, dizziness, or fever occurs during treatment with neratinib.
Hepatotoxicity
- Inform patients that neratinib has been associated with hepatotoxicity which may be severe in some cases.
Inform patients that they should report signs and symptoms of liver dysfunction to their healthcare provider immediately.
Embryo-Fetal Toxicity
- Advise females to inform their healthcare provider if they are pregnant or become pregnant. Inform female patients of the risk to a fetus and potential loss of the pregnancy.
- Advise females of reproductive potential to use effective contraception during treatment and for 1 month after receiving the last dose of neratinib.
- Advise lactating women not to breastfeed during treatment with neratinib and for at least 1 month after the last dose.
Drug Interactions
- Neratinib may interact with many drugs; therefore, advise patients to report to their healthcare provider the use of any other prescription or nonprescription medication or herbal products.
- Neratinib may interact with gastric acid reducing agents. Advise patients to avoid concomitant use of proton pump inhibitors. When patients require gastric acid reducing agents, use an H 2-receptor antagonist or antacid. Advise patients to separate the dosing of neratinib by 3 hours after antacid medicine, and to take neratinib at least 2 hours before or 10 hours after a H 2-receptor antagonist.
- Neratinib may interact with grapefruit. Advise patients to avoid taking neratinib with grapefruit products.
Dosing and Administration
- Instruct patients to take neratinib with food at approximately the same time each day consecutively for one year.
- If a patient misses a dose, instruct the patient not to replace the missed dose, and to resume neratinib with the next scheduled daily dose.
Precautions with Alcohol
Alcohol-Neratinib interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Nerlynx
Look-Alike Drug Names
There is limited information regarding Neratinib Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.