Naproxen and esomeprazole magnesium: Difference between revisions
No edit summary |
No edit summary |
||
| Line 256: | Line 256: | ||
|clinicalTrials= | |clinicalTrials= | ||
*Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | |||
*The adverse reactions reported below are specific to the clinical trials with VIMOVO. See also the full prescribing information for naproxen and esomeprazole magnesium products. | |||
*The safety of VIMOVO was evaluated in clinical studies involving 2317 patients (aged 27 to 90 years) and ranging from 3-12 months. Patients received either 500 mg/20 mg of VIMOVO twice daily (n=1157), 500 mg of enteric-coated naproxen twice daily (n=426), or placebo (n=246). The average number of VIMOVO doses taken over 12 months was 696±44. | |||
*The table below lists all adverse reactions, regardless of causality, occurring in >2% of patients receiving VIMOVO from two clinical studies (Study 1 and Study 2). Both of these studies were randomized, multi-center, double-blind, parallel studies. The majority of patients were female (67%), white (86%). The majority of patients were 50-69 years of age (83%). Approximately one quarter were on low-dose aspirin. | |||
T1 | |||
===== | *In Study 1 and Study 2, patients taking VIMOVO had fewer premature discontinuations due to adverse reactions compared to patients taking enteric-coated naproxen alone (7.9% vs. 12.5% respectively). The most common reasons for discontinuations due to adverse events in the VIMOVO treatment group were upper abdominal pain (1.2%, n=5), duodenal ulcer (0.7%, n=3) and erosive gastritis (0.7%, n=3). Among patients receiving enteric-coated naproxen, the most common reasons for discontinuations due to adverse events were duodenal ulcer 5.4% (n=23), dyspepsia 2.8% (n=12) and upper abdominal pain 1.2% (n=5). The proportion of patients discontinuing treatment due to any upper gastrointestinal adverse events (including duodenal ulcers) in patients treated with VIMOVO was 4% compared to 12% for patients taking enteric-coated naproxen. | ||
*The table below lists all adverse reactions, regardless of causality, occurring in >2% of patients from 2 clinical studies conducted in patients with osteoarthritis of the knee (Study 3 and Study 4). | |||
T2 | |||
*The percentage of subjects who withdrew from the VIMOVO treatment group in these studies due to treatment-emergent adverse events was 7%. There were no preferred terms in which more than 1% of subjects withdrew from any treatment group. | |||
*The long-term safety of VIMOVO was evaluated in an open-label clinical trial of 239 patients, of which 135 patients received 500 mg/20 mg of VIMOVO for 12 months. There were no differences in frequency or types of adverse reactions seen in the long-term safety study compared to shorter-term treatment in the randomized controlled studies. | |||
<!--Postmarketing Experience--> | |||
|postmarketing= | |||
=====Naproxen===== | |||
*The following adverse reactions have been identified during post-approval use of naproxen. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These reports are listed below by body system: | |||
=====Body as a Whole===== | |||
Anaphylactic reactions, angioneurotic edema, menstrual disorders, pyrexia (chills and fever) | |||
=====Cardiovascular===== | |||
Congestive heart failure, vasculitis, hypertension, pulmonary edema | |||
=====Gastrointestinal===== | |||
Gastrointestinal bleeding and/or perforation, hematemesis, pancreatitis, vomiting, colitis, exacerbation of inflammatory bowel disease (ulcerative colitis, Crohn's disease), nonpeptic gastrointestinal ulceration, ulcerative stomatitis, esophagitis, peptic ulceration | |||
=====Hepatobiliary===== | |||
Jaundice, abnormal liver function tests, hepatitis (some cases have been fatal) | |||
=====Hemic and Lymphatic===== | |||
Eosinophilia, leukopenia, melena, thrombocytopenia, agranulocytosis, granulocytopenia, hemolytic anemia, aplastic anemia | |||
=====Metabolic and Nutritional===== | |||
Hyperglycemia, hypoglycemia | |||
=====Nervous System===== | |||
Inability to concentrate, depression, dream abnormalities, insomnia, malaise, myalgia, muscle weakness, aseptic meningitis, cognitive dysfunction, convulsions | |||
=====Respiratory===== | =====Respiratory===== | ||
Eosinophilic pneumonitis, asthma | |||
=====Dermatologic===== | |||
Alopecia, urticaria, skin rashes, toxic epidermal necrolysis, erythema multiforme, erythema nodosum, fixed drug eruption, lichen planus, pustular reaction, systemic lupus erythematoses, bullous reactions, including Stevens-Johnson syndrome, photosensitive dermatitis, photosensitivity reactions, including rare cases resembling porphyria cutanea tarda (pseudoporphyria) or epidermolysis bullosa. If skin fragility, blistering or other symptoms suggestive of pseudoporphyria occur, treatment should be discontinued and the patient monitored. | |||
=====Special Senses===== | =====Special Senses===== | ||
Hearing impairment, corneal opacity, papillitis, retrobulbar optic neuritis, papilledema | |||
=====Urogenital===== | =====Urogenital===== | ||
Glomerular nephritis, hematuria, hyperkalemia, interstitial nephritis, nephrotic syndrome, renal disease, renal failure, renal papillary necrosis, raised serum creatinine | |||
=====Reproduction (female)===== | |||
Infertility | |||
===== | =====Esomeprazole===== | ||
*The following adverse reactions have been identified during post-approval use of esomeprazole. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These reports are listed below by body system: | |||
=====Blood and Lymphatic===== | |||
Agranulocytosis, pancytopenia | |||
=====Eye===== | |||
Blurred vision | |||
===== | =====Gastrointestinal===== | ||
Pancreatitis; stomatitis; microscopic colitis | |||
=====Hepatobiliary===== | |||
Hepatic failure, hepatitis with or without jaundice | |||
=====Immune System===== | |||
Anaphylactic reaction/shock | |||
===== | =====Infections and Infestations===== | ||
GI candidiasis, Clostridium difficile associated diarrhea | |||
=====Metabolism and Nutritional Disorders===== | |||
Hypomagnesemia | |||
=====Musculoskeletal and Connective Tissue===== | |||
Muscular weakness, myalgia, bone fracture | |||
===== | =====Nervous System===== | ||
Hepatic encephalopathy, taste disturbance | |||
=====Psychiatric===== | |||
Aggression, agitation, depression, hallucination | |||
=====Renal and Urinary===== | |||
Interstitial nephritis | |||
===== | =====Reproductive System and Breast===== | ||
Gynecomastia | |||
=====Respiratory, Thoracic, and Mediastinal===== | |||
Bronchospasm | |||
=====Skin and Subcutaneous Tissue===== | |||
Alopecia, erythema multiforme, hyperhidrosis, photosensitivity, Stevens-Johnson syndrome, toxic epidermal necrolysis (some fatal) | |||
<!--Drug Interactions--> | <!--Drug Interactions--> | ||
Revision as of 15:47, 2 December 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: CARDIOVASCULAR AND GASTROINTESTINAL RISKS
See full prescribing information for complete Boxed Warning.
|
Overview
Naproxen and esomeprazole magnesium is a combination of NSAID and proton pump inhibitor that is FDA approved for the {{{indicationType}}} of osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis and to decrease the risk of developing gastric ulcers in patients at risk of developing NSAID associated gastric ulcers. There is a Black Box Warning for this drug as shown here. Common adverse reactions include erosive gastritis, dyspepsia, gastritis, diarrhea, gastric ulcer, upper abdominal pain, and nausea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Rheumatoid Arthritis
- Dosing Information
- The dosage is one tablet twice daily of VIMOVO 375 mg naproxen and 20 mg of esomeprazole or 500 mg naproxen and 20 mg of esomeprazole.
Osteoarthritis
- Dosing Information
- The dosage is one tablet twice daily of VIMOVO 375 mg naproxen and 20 mg of esomeprazole or 500 mg naproxen and 20 mg of esomeprazole.
Ankylosing Spondylitis
- Dosing Information
- The dosage is one tablet twice daily of VIMOVO 375 mg naproxen and 20 mg of esomeprazole or 500 mg naproxen and 20 mg of esomeprazole.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Naproxen and esomeprazole magnesium in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Naproxen and esomeprazole magnesium in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Naproxen and esomeprazole magnesium in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Naproxen and esomeprazole magnesium in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Naproxen and esomeprazole magnesium in pediatric patients.
Contraindications
- VIMOVO is contraindicated in patients with known hypersensitivity to naproxen, esomeprazole magnesium, substituted benzimidazoles, or to any of the excipients.
- VIMOVO is contraindicated in patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs. Severe, rarely fatal, anaphylactic-like reactions to NSAIDs have been reported in such patients. Hypersensitivity reactions, e.g., angioedema and anaphylactic reaction/shock, have been reported with esomeprazole use.
- VIMOVO is contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery.
Warnings
|
WARNING: CARDIOVASCULAR AND GASTROINTESTINAL RISKS
See full prescribing information for complete Boxed Warning.
|
Precautions
- Cardiovascular Thrombotic Events
- Clinical trials of several COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of serious cardiovascular (CV) thrombotic events, myocardial infarction, and stroke, which can be fatal. All NSAIDS, both COX-2 selective and nonselective, may have a similar risk. Patients with known CV disease or risk factors for CV disease may be at greater risk. To minimize the potential risk for an adverse CV event in patients treated with an NSAID, the lowest effective dose should be used for the shortest duration possible. Physicians and patients should remain alert for the development of such events, even in the absence of previous CV symptoms. Patients should be informed about the signs and/or symptoms of serious CV events and the steps to take if they occur.
- There is no consistent evidence that concurrent use of aspirin mitigates the increased risk of serious CV thrombotic events associated with NSAID use.
- Two large, controlled, clinical trials of a COX-2 selective NSAID for the treatment of pain in the first 10–14 days following CABG surgery found an increased incidence of myocardial infarction and stroke.
- Hypertension
- NSAIDs, including naproxen, a component of VIMOVO, can lead to onset of new hypertension or worsening of pre-existing hypertension, either of which may contribute to the increased incidence of CV events. Patients taking thiazides or loop diuretics may have impaired response to these therapies when taking NSAIDs. NSAIDs should be used with caution in patients with hypertension. Blood pressure (BP) should be monitored closely during the initiation of NSAID treatment and throughout the course of therapy.
- Congestive Heart Failure and Edema
- Fluid retention, edema, and peripheral edema have been observed in some patients taking NSAIDs and should be used with caution in patients with fluid retention or heart failure.
- Gastrointestinal Effects — Risk of Ulceration, Bleeding, and Perforation
- NSAIDs, including naproxen, a component of VIMOVO, can cause serious gastrointestinal (GI) adverse events including inflammation, bleeding, ulceration, and perforation of the stomach, small intestine, or large intestine, which can be fatal. While VIMOVO has been shown to significantly decrease the occurrence of gastric ulcers compared to naproxen alone, ulceration and associated complications can still occur.
- These serious adverse events can occur at any time, with or without warning symptoms, in patients treated with NSAIDs. Only one in five patients who develop a serious upper GI adverse event on NSAID therapy is symptomatic. Upper GI ulcers, gross bleeding, or perforation caused by NSAIDs occur in approximately 1% of patients treated for 3–6 months, and in about 2–4% of patients treated for one year. These trends continue with longer duration of use, increasing the likelihood of developing a serious GI event at some time during the course of therapy. However, even short-term therapy is not without risk. The utility of periodic laboratory monitoring has not been demonstrated, nor has it been adequately assessed.
- VIMOVO should be prescribed with caution in those with a prior history of ulcer disease or gastrointestinal bleeding. Patients with a prior history of peptic ulcer disease and/or gastrointestinal bleeding who use NSAIDs have a greater than 10-fold increased risk of developing a GI bleed compared to patients with neither of these risk factors. Other factors that increase the risk for GI bleeding in patients treated with NSAIDs include concomitant use of oral corticosteroids or anticoagulants or antiplatelets (including low-dose aspirin), longer duration of NSAID therapy, smoking, use of alcohol, older age, and poor general health status. Most spontaneous reports of fatal GI events are in elderly or debilitated patients, and therefore special care should be taken in treating this population.
- To minimize the potential risk for an adverse GI event in patients treated with an NSAID or NSAID-containing product, the lowest effective dose should be used for the shortest possible duration. Patients and physicians should remain alert for signs and symptoms of GI ulceration and bleeding during NSAID therapy and promptly initiate additional evaluation and treatment if a serious GI adverse event is suspected. This should include discontinuation of the NSAID until a serious GI adverse event is ruled out. For high risk patients, alternate therapies that do not involve NSAIDs should be considered.
- Epidemiological studies of the case-control and cohort design have demonstrated an association between use of psychotropic drugs that interfere with serotonin reuptake and the occurrence of upper gastrointestinal bleeding. In two studies, concurrent use of an NSAID, COX-2 inhibitor, or aspirin potentiated the risk of bleeding. Although these studies focused on upper gastrointestinal bleeding, bleeding at other sites cannot be ruled out.
- NSAIDs should be given with care to patients with a history of inflammatory bowel disease (ulcerative colitis, Crohn's disease) as their condition may be exacerbated.
- Gastrointestinal symptomatic response to therapy with VIMOVO does not preclude the presence of gastric malignancy.
- Atrophic gastritis has been noted occasionally in gastric corpus biopsies from patients treated long-term with omeprazole, of which esomeprazole is an enantiomer and a component of VIMOVO.
- Active Bleeding
- When active and clinically significant bleeding from any source occurs in patients receiving VIMOVO, the treatment should be withdrawn.
- Renal Effects
- Long-term administration of NSAIDs has resulted in renal papillary necrosis and other renal injury. Renal toxicity has also been seen in patients in whom renal prostaglandins have a compensatory role in the maintenance of renal perfusion. In these patients, administration of an NSAID may cause a dose-dependent reduction in prostaglandin formation and, secondarily, in renal blood flow, which may precipitate overt renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, hypovolemia, heart failure, liver dysfunction, salt depletion, those taking diuretics, ACE inhibitors, or angiotensin II receptor antagonists and the elderly. Discontinuation of NSAID therapy is usually followed by recovery to the pretreatment state.
- Advanced Renal Disease
- No information is available from controlled clinical studies regarding the use of VIMOVO in patients with advanced renal disease. Therefore, treatment with VIMOVO is not recommended in these patients with advanced renal disease. If VIMOVO therapy must be initiated, close monitoring of the patient's renal function is advisable.
- Anaphylactic Reactions
- Anaphylactic reactions may occur in patients without known prior exposure to either component of VIMOVO. NSAIDs should not be given to patients with the aspirin triad. This symptom complex typically occurs in asthmatic patients who experience rhinitis with or without nasal polyps, or who exhibit severe, potentially fatal bronchospasm after taking aspirin or other NSAIDs. Emergency help should be sought in cases where an anaphylactic reaction occurs. Anaphylactic reactions, like anaphylaxis, may have a fatal outcome.
- Skin Reactions
- NSAIDs can cause serious skin adverse events such as exfoliative dermatitis, Stevens-Johnson syndrome, and toxic epidermal necrolysis, which can be fatal. These serious events may occur without warning. Patients should be informed about the signs and symptoms of serious skin manifestations and use of the drug should be discontinued at the first appearance of skin rash or any other sign of hypersensitivity.
- Fetal Toxicity
- Starting at 30 weeks gestation, VIMOVO, as with other NSAID-containing products, should be avoided because it may cause premature closure of the ductus arteriosus.
- Hepatic Effects
- Borderline elevations of one or more liver tests may occur in up to 15% of patients taking NSAIDs including naproxen, a component of VIMOVO. Hepatic abnormalities may be the result of hypersensitivity rather than direct toxicity. These laboratory abnormalities may progress, may remain essentially unchanged, or may be transient with continued therapy. The SGPT (ALT) test is probably the most sensitive indicator of liver dysfunction. Notable elevations of ALT or AST (approximately three or more times the upper limit of normal) have been reported in approximately 1% of patients in clinical trials with NSAIDs. In addition, rare cases of severe hepatic reactions, including jaundice and fatal fulminant hepatitis, liver necrosis and hepatic failure, some of them with fatal outcomes, have been reported.
- A patient with symptoms and/or signs suggesting liver dysfunction, or in whom an abnormal liver test has occurred, should be evaluated for evidence of the development of more severe hepatic reaction while on therapy with VIMOVO.
- If clinical signs and symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g., eosinophilia, rash, etc.), VIMOVO should be discontinued.
- Chronic alcoholic liver disease and probably other diseases with decreased or abnormal plasma proteins (albumin) reduce the total plasma concentration of naproxen, but the plasma concentration of unbound naproxen is increased. Caution is advised when high doses are required and some adjustment of dosage may be required in these patients. It is prudent to use the lowest effective dose for the shortest possible duration of adequate treatment.
- VIMOVO should be avoided in patients with severe hepatic impairment.
- Hematological Effects
- Anemia is sometimes seen in patients receiving NSAIDs. This may be due to fluid retention, occult or gross GI blood loss, or an incompletely described effect upon erythropoiesis. Patients on long-term treatment with NSAIDs should have their hemoglobin or hematocrit checked if they exhibit any signs or symptoms of anemia.
- NSAIDs inhibit platelet aggregation and have been shown to prolong bleeding time in some patients. Unlike aspirin, their effect on platelet function is quantitatively less, of shorter duration, and reversible. Patients receiving VIMOVO who may be adversely affected by alterations in platelet function, such as those with coagulation disorders or patients receiving anticoagulants or antiplatelets, should be carefully monitored.
- Pre-existing Asthma
- Patients with asthma may have aspirin-sensitive asthma. The use of aspirin in patients with aspirin-sensitive asthma has been associated with severe bronchospasm, which can be fatal. Since cross reactivity, including bronchospasm, between aspirin and other NSAIDs has been reported in such aspirin-sensitive patients, VIMOVO should not be administered to patients with this form of aspirin sensitivity and should be used with caution in patients with pre-existing asthma.
- Concomitant NSAID Use
- VIMOVO contains naproxen as one of its active ingredients. It should not be used with other naproxen-containing products since they all circulate in the plasma as the naproxen anion.
- The concomitant use of VIMOVO with any dose of a non-aspirin NSAID should be avoided due to the potential for increased risk of adverse reactions.
- Corticosteroid Treatment
- VIMOVO cannot be expected to substitute for corticosteroids or to treat corticosteroid insufficiency. Abrupt discontinuation of corticosteroids may lead to disease exacerbation. Patients on prolonged corticosteroid therapy should have their therapy tapered slowly if a decision is made to discontinue corticosteroids and the patient should be observed closely for any evidence of adverse effects, including adrenal insufficiency and exacerbation of symptoms of arthritis.
- Clostridium difficile associated diarrhea
- Published observational studies suggest that proton pump inhibitor (PPI) therapy like VIMOVO may be associated with an increased risk of Clostridium difficile associated diarrhea, especially in hospitalized patients. This diagnosis should be considered for diarrhea that does not improve.
- Patients should use the lowest dose and shortest duration of PPI therapy appropriate to the condition being treated.
- Interaction with Clopidogrel
- Avoid concomitant use of esomeprazole with clopidogrel. Clopidogrel is a prodrug. Inhibition of platelet aggregation by clopidogrel is entirely due to an active metabolite. The metabolism of clopidogrel to its active metabolite can be impaired by use with concomitant medications, such as esomeprazole, that inhibit CYP2C19 activity. Concomitant use of clopidogrel with 40 mg esomeprazole reduces the pharmacological activity of clopidogrel. When using esomeprazole, a component of VIMOVO, consider alternative anti-platelet therapy.
- Bone Fracture
- Several published observational studies suggest that PPI therapy may be associated with an increased risk for osteoporosis-related fractures of the hip, wrist, or spine. The risk of fracture was increased in patients who received high-dose, defined as multiple daily doses, and long-term PPI therapy (a year or longer). Patients should use the lowest dose and shortest duration of PPI therapy appropriate to the condition being treated. Patients at risk for osteoporosis-related fractures should be managed according to the established treatment guidelines.
- VIMOVO (a combination PPI/NSAID) is approved for use twice a day and does not allow for administration of a lower daily dose of the PPI.
- Masking of Inflammation and Fever
- The pharmacological activity of VIMOVO in reducing fever and inflammation may diminish the utility of these diagnostic signs in detecting complications of presumed noninfectious, noninflammatory painful conditions.
- Laboratory Tests
- Because serious GI tract ulcerations and bleeding can occur without warning symptoms, physicians should monitor for signs or symptoms of GI bleeding. Patients on long-term treatment with NSAIDs should have their CBC and a chemistry profile checked periodically. If clinical signs and symptoms consistent with liver or renal disease develop, systemic manifestations occur (e.g., eosinophilia, rash, etc.) or if abnormal liver tests persist or worsen, VIMOVO should be discontinued.
- Patients with initial hemoglobin values of 10 g or less who are to receive long-term therapy should have hemoglobin values determined periodically.
- Serum chromogranin A (CgA) levels increase secondary to drug-induced decreases in gastric acidity. The increased CgA level may cause false positive results in diagnostic investigations for neuroendocrine tumors. Providers should temporarily stop esomeprazole treatment at least 14 days before assessing CgA levels and consider repeating the test if initial CgA levels are high. If serial tests are performed (e.g. for monitoring), the same commercial laboratory should be used for testing, as reference ranges between tests may vary.
- Hypomagnesemia
- Hypomagnesemia, symptomatic and asymptomatic, has been reported rarely in patients treated with PPIs for at least three months, in most cases after a year of therapy. Serious adverse events include tetany, arrhythmias, and seizures. In most patients, treatment of hypomagnesemia required magnesium replacement and discontinuation of the PPI.
- For patients expected to be on prolonged treatment or who take PPIs with medications such as digoxin or drugs that may cause hypomagnesemia (e.g., diuretics), health care professionals may consider monitoring magnesium levels prior to initiation of PPI treatment and periodically.
- Concomitant use of St John's Wort or Rifampin with VIMOVO
- Drugs that induce CYP2C19 or CYP3A4 (such as St John's Wort or rifampin) can substantially decrease esomeprazole concentrations. Avoid concomitant use of VIMOVO with St John's Wort or rifampin.
- Concomitant use of VIMOVO with Methotrexate
- Literature suggests that concomitant use of PPIs with methotrexate (primarily at high dose; see methotrexate prescribing information) may elevate and prolong serum levels of methotrexate and/or its metabolite, possibly leading to methotrexate toxicities. In high-dose methotrexate administration a temporary withdrawal of the PPI may be considered in some patients.
Adverse Reactions
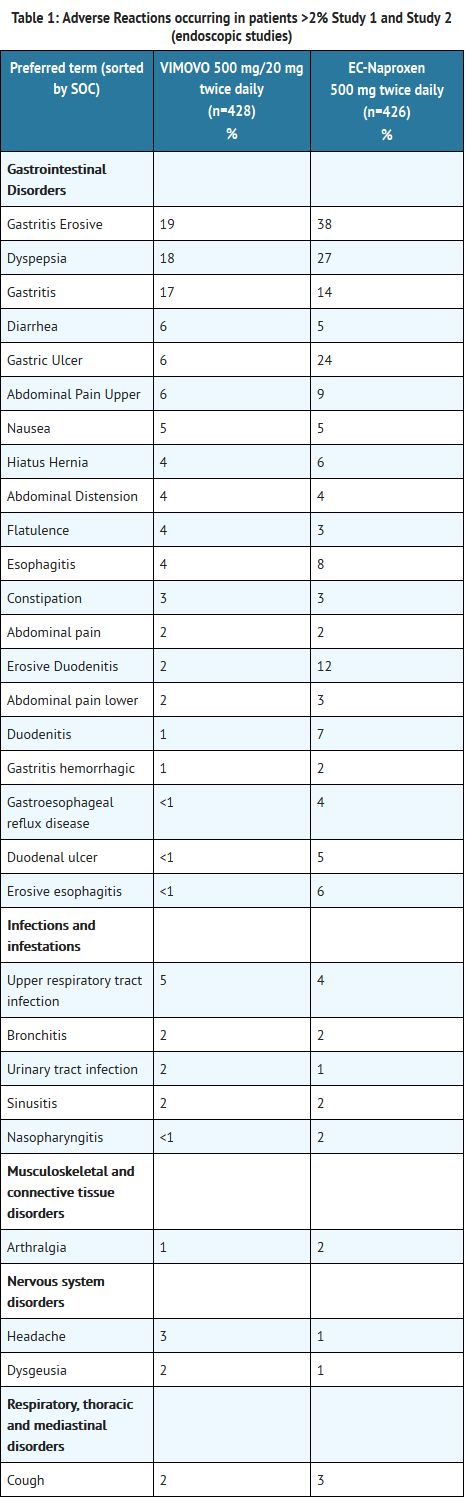
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The adverse reactions reported below are specific to the clinical trials with VIMOVO. See also the full prescribing information for naproxen and esomeprazole magnesium products.
- The safety of VIMOVO was evaluated in clinical studies involving 2317 patients (aged 27 to 90 years) and ranging from 3-12 months. Patients received either 500 mg/20 mg of VIMOVO twice daily (n=1157), 500 mg of enteric-coated naproxen twice daily (n=426), or placebo (n=246). The average number of VIMOVO doses taken over 12 months was 696±44.
- The table below lists all adverse reactions, regardless of causality, occurring in >2% of patients receiving VIMOVO from two clinical studies (Study 1 and Study 2). Both of these studies were randomized, multi-center, double-blind, parallel studies. The majority of patients were female (67%), white (86%). The majority of patients were 50-69 years of age (83%). Approximately one quarter were on low-dose aspirin.
T1
- In Study 1 and Study 2, patients taking VIMOVO had fewer premature discontinuations due to adverse reactions compared to patients taking enteric-coated naproxen alone (7.9% vs. 12.5% respectively). The most common reasons for discontinuations due to adverse events in the VIMOVO treatment group were upper abdominal pain (1.2%, n=5), duodenal ulcer (0.7%, n=3) and erosive gastritis (0.7%, n=3). Among patients receiving enteric-coated naproxen, the most common reasons for discontinuations due to adverse events were duodenal ulcer 5.4% (n=23), dyspepsia 2.8% (n=12) and upper abdominal pain 1.2% (n=5). The proportion of patients discontinuing treatment due to any upper gastrointestinal adverse events (including duodenal ulcers) in patients treated with VIMOVO was 4% compared to 12% for patients taking enteric-coated naproxen.
- The table below lists all adverse reactions, regardless of causality, occurring in >2% of patients from 2 clinical studies conducted in patients with osteoarthritis of the knee (Study 3 and Study 4).
T2
- The percentage of subjects who withdrew from the VIMOVO treatment group in these studies due to treatment-emergent adverse events was 7%. There were no preferred terms in which more than 1% of subjects withdrew from any treatment group.
- The long-term safety of VIMOVO was evaluated in an open-label clinical trial of 239 patients, of which 135 patients received 500 mg/20 mg of VIMOVO for 12 months. There were no differences in frequency or types of adverse reactions seen in the long-term safety study compared to shorter-term treatment in the randomized controlled studies.
Postmarketing Experience
Naproxen
- The following adverse reactions have been identified during post-approval use of naproxen. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These reports are listed below by body system:
Body as a Whole
Anaphylactic reactions, angioneurotic edema, menstrual disorders, pyrexia (chills and fever)
Cardiovascular
Congestive heart failure, vasculitis, hypertension, pulmonary edema
Gastrointestinal
Gastrointestinal bleeding and/or perforation, hematemesis, pancreatitis, vomiting, colitis, exacerbation of inflammatory bowel disease (ulcerative colitis, Crohn's disease), nonpeptic gastrointestinal ulceration, ulcerative stomatitis, esophagitis, peptic ulceration
Hepatobiliary
Jaundice, abnormal liver function tests, hepatitis (some cases have been fatal)
Hemic and Lymphatic
Eosinophilia, leukopenia, melena, thrombocytopenia, agranulocytosis, granulocytopenia, hemolytic anemia, aplastic anemia
Metabolic and Nutritional
Hyperglycemia, hypoglycemia
Nervous System
Inability to concentrate, depression, dream abnormalities, insomnia, malaise, myalgia, muscle weakness, aseptic meningitis, cognitive dysfunction, convulsions
Respiratory
Eosinophilic pneumonitis, asthma
Dermatologic
Alopecia, urticaria, skin rashes, toxic epidermal necrolysis, erythema multiforme, erythema nodosum, fixed drug eruption, lichen planus, pustular reaction, systemic lupus erythematoses, bullous reactions, including Stevens-Johnson syndrome, photosensitive dermatitis, photosensitivity reactions, including rare cases resembling porphyria cutanea tarda (pseudoporphyria) or epidermolysis bullosa. If skin fragility, blistering or other symptoms suggestive of pseudoporphyria occur, treatment should be discontinued and the patient monitored.
Special Senses
Hearing impairment, corneal opacity, papillitis, retrobulbar optic neuritis, papilledema
Urogenital
Glomerular nephritis, hematuria, hyperkalemia, interstitial nephritis, nephrotic syndrome, renal disease, renal failure, renal papillary necrosis, raised serum creatinine
Reproduction (female)
Infertility
Esomeprazole
- The following adverse reactions have been identified during post-approval use of esomeprazole. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These reports are listed below by body system:
Blood and Lymphatic
Agranulocytosis, pancytopenia
Eye
Blurred vision
Gastrointestinal
Pancreatitis; stomatitis; microscopic colitis
Hepatobiliary
Hepatic failure, hepatitis with or without jaundice
Immune System
Anaphylactic reaction/shock
Infections and Infestations
GI candidiasis, Clostridium difficile associated diarrhea
Metabolism and Nutritional Disorders
Hypomagnesemia
Musculoskeletal and Connective Tissue
Muscular weakness, myalgia, bone fracture
Nervous System
Hepatic encephalopathy, taste disturbance
Psychiatric
Aggression, agitation, depression, hallucination
Renal and Urinary
Interstitial nephritis
Reproductive System and Breast
Gynecomastia
Respiratory, Thoracic, and Mediastinal
Bronchospasm
Skin and Subcutaneous Tissue
Alopecia, erythema multiforme, hyperhidrosis, photosensitivity, Stevens-Johnson syndrome, toxic epidermal necrolysis (some fatal)
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Naproxen and esomeprazole magnesium in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Naproxen and esomeprazole magnesium during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Naproxen and esomeprazole magnesium with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Naproxen and esomeprazole magnesium with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Naproxen and esomeprazole magnesium with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Naproxen and esomeprazole magnesium with respect to specific gender populations.
Race
There is no FDA guidance on the use of Naproxen and esomeprazole magnesium with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Naproxen and esomeprazole magnesium in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Naproxen and esomeprazole magnesium in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Naproxen and esomeprazole magnesium in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Naproxen and esomeprazole magnesium in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Naproxen and esomeprazole magnesium in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Naproxen and esomeprazole magnesium in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Naproxen and esomeprazole magnesium in the drug label.
Pharmacology
There is limited information regarding Naproxen and esomeprazole magnesium Pharmacology in the drug label.
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Naproxen and esomeprazole magnesium in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Naproxen and esomeprazole magnesium in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Naproxen and esomeprazole magnesium in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Naproxen and esomeprazole magnesium in the drug label.
How Supplied
Storage
There is limited information regarding Naproxen and esomeprazole magnesium Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Naproxen and esomeprazole magnesium |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Naproxen and esomeprazole magnesium |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Naproxen and esomeprazole magnesium in the drug label.
Precautions with Alcohol
- Alcohol-Naproxen and esomeprazole magnesium interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Naproxen and esomeprazole magnesium |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Naproxen and esomeprazole magnesium |Label Name=Naproxen and esomeprazole magnesium11.png
}}
{{#subobject:
|Label Page=Naproxen and esomeprazole magnesium |Label Name=Naproxen and esomeprazole magnesium11.png
}}