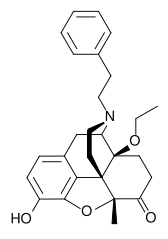
N-Phenethyl-14-ethoxymetopon
 | |
| Identifiers | |
|---|---|
| |
| PubChem CID | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C27H31NO4 |
| Molar mass | 433.538 g/mol |
| 3D model (JSmol) | |
| |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [2] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
N-Phenethyl-14-ethoxymetopon is a drug which is a derivative of metopon. It is a potent analgesic, around 60 times stronger than morphine, but produces significantly less constipation.[1]
N-Phenethyl-14-ethoxymetopon acts as an agonist at both μ- and δ-opioid receptors, with a Ki of 0.16nM at μ and 3.14nM at δ.[2]
References
- ↑ Ananthan S. Opioid ligands with mixed mu/delta opioid receptor interactions: an emerging approach to novel analgesics. AAPS Journal. 2006 Mar 10;8(1):E118-25. PMID 16584118
- ↑ Lattanzi R, Spetea M, Schüllner F, Rief SB, Krassnig R, Negri L, Schmidhammer H. Synthesis and biological evaluation of 14-alkoxymorphinans. 22.(1) Influence of the 14-alkoxy group and the substitution in position 5 in 14-alkoxymorphinan-6-ones on in vitro and in vivo activities. Journal of Medicinal Chemistry. 2005 May 5;48(9):3372-8. PMID 15857143
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs missing an ATC code
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Opioids
- Delta-opioid agonists