Myeloproliferative neoplasm pathophysiology: Difference between revisions
Gerald Chi (talk | contribs) m (→Myelofibrosis) |
Gerald Chi (talk | contribs) mNo edit summary |
||
| Line 15: | Line 15: | ||
====Chronic Myelogenous Leukemia==== | ====Chronic Myelogenous Leukemia==== | ||
{{ | {{Main|Chronic myelogenous leukemia pathophysiology}} | ||
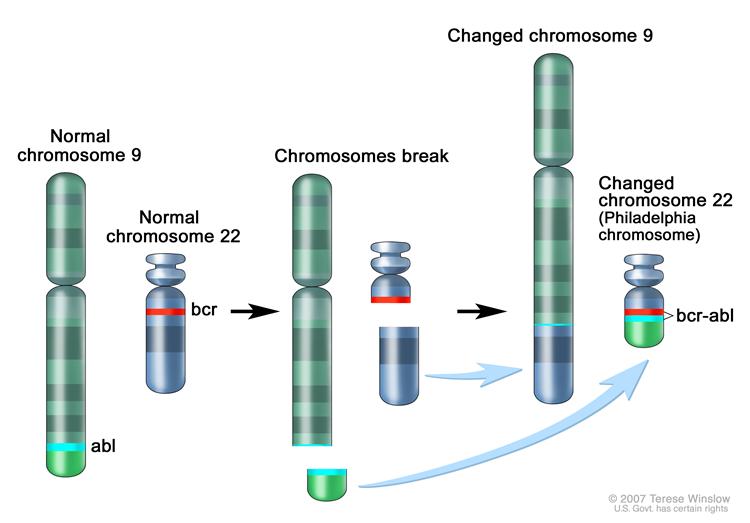
In [[chronic myelogenous leukemia]], the [[Philadelphia chromosome|Phialdelphia chromosome]] results from a balanced reciprocal translocation between chromosomes 9 and 22 termed t(9;22)(q34;q11.2). This leads to formation of a hybrid BCR-ABL gene which encodes for an oncoprotein p210<sup>BCR-ABL</sup> with a constitutive tyrosine kinase activity. Activation of the BCR-ABL oncogene and downstream signaling pathways confers survival advantage to leukemic cells and suppresses normal [[hematopoiesis]]. | In [[chronic myelogenous leukemia]], the [[Philadelphia chromosome|Phialdelphia chromosome]] results from a balanced reciprocal translocation between chromosomes 9 and 22 termed t(9;22)(q34;q11.2). This leads to formation of a hybrid BCR-ABL gene which encodes for an oncoprotein p210<sup>BCR-ABL</sup> with a constitutive tyrosine kinase activity. Activation of the BCR-ABL oncogene and downstream signaling pathways confers survival advantage to leukemic cells and suppresses normal [[hematopoiesis]]. | ||
====Polycythemia Vera==== | ====Polycythemia Vera==== | ||
{{ | {{Main|Polycythemia vera pathophysiology}} | ||
In [[polycythemia vera]], the erythroid progenitors display growth factor hypersensitivity to [[insulin-like growth factor-1]] (IGF-1) and other cytokines. Mutations in the Janus kinase 2 gene, JAK2V617V in particular, also contribute to the constitutive activation and cytokine-independent proliferation of [[CD34]]+ hematopoietic progenitors and their progeny. | In [[polycythemia vera]], the erythroid progenitors display growth factor hypersensitivity to [[insulin-like growth factor-1]] (IGF-1) and other cytokines. Mutations in the Janus kinase 2 gene, JAK2V617V in particular, also contribute to the constitutive activation and cytokine-independent proliferation of [[CD34]]+ hematopoietic progenitors and their progeny. | ||
====Primary Myelofibrosis==== | ====Primary Myelofibrosis==== | ||
{{ | {{Main|Myelofibrosis pathophysiology}} | ||
Primary myelofibrosis, hallmarked by clonal myeloproliferation with reactive stromal aberration, reflects an uncontrolled production of growth factors (e.g., [[TGF beta|transforming growth factor β]], [[platelet-derived growth factor|platelet-derived growth factor]], and [[basic fibroblast growth factor|basic fibroblast growth factor]]) from resident megakaryocyes and monocytes. Etiopathogenic mutations leading to primary myelofibrosis remain unclear.<ref>{{cite book | last = Jaffe | first = Elaine | title = Pathology and genetics of tumours of haematopoietic and lymphoid tissues | publisher = IARC Press Oxford University Press | year = 2001 | isbn = 978-9283224112 }}</ref> | Primary myelofibrosis, hallmarked by clonal myeloproliferation with reactive stromal aberration, reflects an uncontrolled production of growth factors (e.g., [[TGF beta|transforming growth factor β]], [[platelet-derived growth factor|platelet-derived growth factor]], and [[basic fibroblast growth factor|basic fibroblast growth factor]]) from resident megakaryocyes and monocytes. Etiopathogenic mutations leading to primary myelofibrosis remain unclear.<ref>{{cite book | last = Jaffe | first = Elaine | title = Pathology and genetics of tumours of haematopoietic and lymphoid tissues | publisher = IARC Press Oxford University Press | year = 2001 | isbn = 978-9283224112 }}</ref> | ||
Revision as of 20:40, 1 April 2015
|
Myeloproliferative Neoplasm Microchapters |
|
Differentiating myeloproliferative neoplasm from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Myeloproliferative neoplasm pathophysiology On the Web |
|
American Roentgen Ray Society Images of Myeloproliferative neoplasm pathophysiology |
|
Directions to Hospitals Treating Myeloproliferative neoplasm |
|
Risk calculators and risk factors for Myeloproliferative neoplasm pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Clinical and pathologic findings in the myeloproliferative neoplasms (MPNs) are due to dysregulated proliferation and expansion of myeloid progenitors in the bone marrow, resulting in altered populations of granulocytes, erythrocytes, or platelets in the peripheral blood.
Pathophysiology
 |
Genetics
Although no specific genetic abnormalities or disease-initiating events have yet been identified for most of the MPNs, abnormal activation of tyrosine kinase-dependent signal transduction pathways are frequently implicated in their pathogenesis.
Chronic Myelogenous Leukemia
In chronic myelogenous leukemia, the Phialdelphia chromosome results from a balanced reciprocal translocation between chromosomes 9 and 22 termed t(9;22)(q34;q11.2). This leads to formation of a hybrid BCR-ABL gene which encodes for an oncoprotein p210BCR-ABL with a constitutive tyrosine kinase activity. Activation of the BCR-ABL oncogene and downstream signaling pathways confers survival advantage to leukemic cells and suppresses normal hematopoiesis.
Polycythemia Vera
In polycythemia vera, the erythroid progenitors display growth factor hypersensitivity to insulin-like growth factor-1 (IGF-1) and other cytokines. Mutations in the Janus kinase 2 gene, JAK2V617V in particular, also contribute to the constitutive activation and cytokine-independent proliferation of CD34+ hematopoietic progenitors and their progeny.
Primary Myelofibrosis
Primary myelofibrosis, hallmarked by clonal myeloproliferation with reactive stromal aberration, reflects an uncontrolled production of growth factors (e.g., transforming growth factor β, platelet-derived growth factor, and basic fibroblast growth factor) from resident megakaryocyes and monocytes. Etiopathogenic mutations leading to primary myelofibrosis remain unclear.[2]
References
- ↑ "Chronic Myelogenous Leukemia Treatment".
- ↑ Jaffe, Elaine (2001). Pathology and genetics of tumours of haematopoietic and lymphoid tissues. IARC Press Oxford University Press. ISBN 978-9283224112.