Mitomycin (patient information)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Stefano Giannoni [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Mitomycin (patient information) is {{{aOrAn}}} {{{drugClass}}} that is FDA approved for the {{{indicationType}}} of {{{indication}}}. Common adverse reactions include {{{adverseReactions}}}.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Mitomycin (patient information) FDA-Labeled Indications and Dosage (Adult) in the drug label.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Mitomycin (patient information) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Mitomycin (patient information) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Mitomycin (patient information) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Mitomycin (patient information) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Mitomycin (patient information) in pediatric patients.
Contraindications
There is limited information regarding Mitomycin (patient information) Contraindications in the drug label.
Warnings
There is limited information regarding Mitomycin (patient information) Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Mitomycin (patient information) Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Mitomycin (patient information) Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Mitomycin (patient information) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Mitomycin (patient information) in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Mitomycin (patient information) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Mitomycin (patient information) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Mitomycin (patient information) in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Mitomycin (patient information) in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Mitomycin (patient information) in geriatric settings.
Gender
There is no FDA guidance on the use of Mitomycin (patient information) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Mitomycin (patient information) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Mitomycin (patient information) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Mitomycin (patient information) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Mitomycin (patient information) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Mitomycin (patient information) in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Mitomycin (patient information) Administration in the drug label.
Monitoring
There is limited information regarding Mitomycin (patient information) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Mitomycin (patient information) and IV administrations.
Overdosage
There is limited information regarding Mitomycin (patient information) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Mitomycin (patient information) Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Mitomycin (patient information) Mechanism of Action in the drug label.
Structure
There is limited information regarding Mitomycin (patient information) Structure in the drug label.
Pharmacodynamics
There is limited information regarding Mitomycin (patient information) Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Mitomycin (patient information) Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Mitomycin (patient information) Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Mitomycin (patient information) Clinical Studies in the drug label.
How Supplied
There is limited information regarding Mitomycin (patient information) How Supplied in the drug label.
Storage
There is limited information regarding Mitomycin (patient information) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Mitomycin (patient information) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

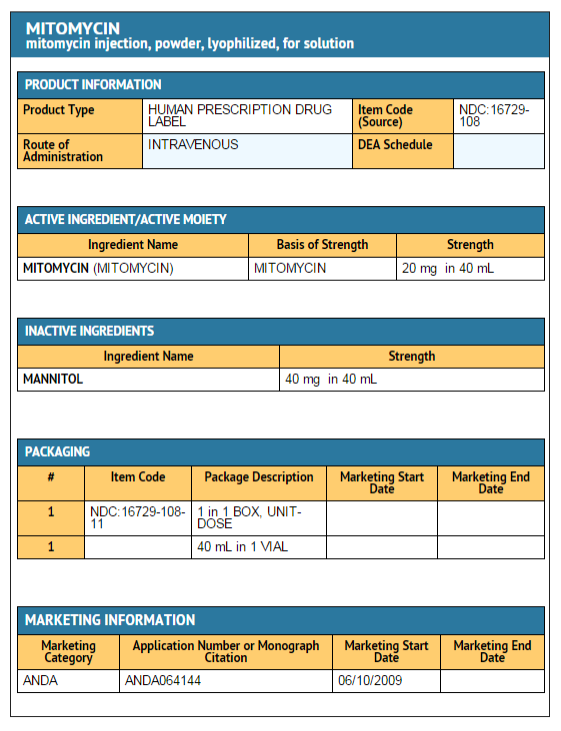
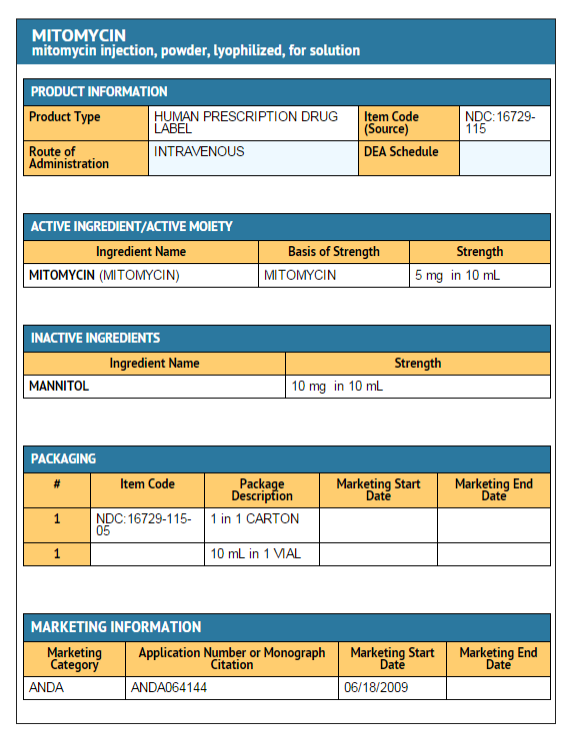
{{#ask: Label Page::Mitomycin (patient information) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Mitomycin (patient information) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Mitomycin (patient information) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Mutamycin[1]
Look-Alike Drug Names
There is limited information regarding Mitomycin (patient information) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Mitomycin (patient information) |Label Name=Mitomycin Package.png
}}
{{#subobject:
|Label Page=Mitomycin (patient information) |Label Name=Mitomycin Package 2.png
}}
{{#subobject:
|Label Page=Mitomycin (patient information) |Label Name=Mitomycin Package 3.png
}}
IMPORTANT WARNING:
Mitomycin can cause a decrease in the number of blood cells in your bone marrow. Mitomycin also can cause kidney damage. Your doctor will order certain lab tests to check your response to mitomycin.
About your treatment
Your doctor has ordered the drug mitomycin to help treat your illness. The drug is given by injection into a vein.
This medication is used to treat:
- adenocarcinoma of the stomach and pancreas
This medication is sometimes prescribed for other uses; ask your doctor or pharmacist for more information.
Mitomycin is a type of antibiotic that is only used in cancer chemotherapy. It slows or stops the growth of cancer cells in your body. The length of treatment depends on the types of drugs you are taking, how well your body responds to them, and the type of cancer you have.
Other uses for this medicine
Mitomycin is also used to treat adenocarcinoma of the colon and rectum; squamous cell carcinoma of the head and neck, lungs, and cervix; adenocarcinoma and duct cell carcinoma of the breast; and bladder cancer. Talk to your doctor about the possible risks of using this drug for your condition.
Precautions
Before taking mitomycin
- tell your doctor and pharmacist if you are allergic to mitomycin or any other drugs.
- tell your doctor and pharmacist what prescription and nonprescription medications you are taking, especially aspirin and vitamins.
- tell your doctor if you have or have ever had kidney disease.
you should know that mitomycin may interfere with the normal menstrual cycle (period) in women and may stop sperm production in men. However, you should not assume that you cannot get pregnant or that you cannot get someone else pregnant. Women who are pregnant or breast-feeding should tell their doctors before they begin taking this drug. You should not plan to have children while receiving chemotherapy or for a while after treatments. (Talk to your doctor for further details.) Use a reliable method of birth control to prevent pregnancy. Mitomycin may harm the fetus.
- do not have any vaccinations (e.g., measles or flu shots) without talking to your doctor.
Side effects
Mild side effects
Side effects from mitomycin are common and include:
- nausea and vomiting
- loss of appetite
- thinned or brittle hair
Severe side effects
Tell your doctor if either of these symptoms is severe or lasts for several hours:
- fatigue or weakness
- mouth blistering
If you experience any of the following symptoms, call your doctor immediately:
- unusual bruising or bleeding
- pain, redness, or swelling at the injection site
- fever
- chills
- sore throat
- cough
- rash
- itching
- difficulty urinating
- swelling of the ankles or feet
- dizziness
- shortness of breath or difficulty breathing
If you experience a serious side effect, you or your doctor may send a report to the Food and Drug Administration's (FDA) MedWatch Adverse Event Reporting program online [at http://www.fda.gov/MedWatch/report.htm] or by phone [1-800-332-1088].
In case of emergency/overdose
In case of overdose, call your local poison control center at 1-800-222-1222. If the victim has collapsed or is not breathing, call local emergency services at 911.
Brand names
- Mutamycin®
Other names
- Mitomycin-C