Migraine pathophysiology
|
Migraine Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Migraine pathophysiology On the Web |
|
American Roentgen Ray Society Images of Migraine pathophysiology |
|
Risk calculators and risk factors for Migraine pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Migraines are believed to be a neurovascular disorder[1][2] with evidence supporting its mechanisms starting within the brain and then spreading to the blood vessels.[3] Migraine begins by neuronal changes leading to the activation of the brainstem and diencephalic nuclei and subsequent dilatation of the large cranial and proximal intracranial vessels.[1] Some researchers feel neuronal mechanisms play a greater role,[4] while others feel blood vessels play the key role.[5] Others feel both are likely important.[6] High levels of the neurotransmitter serotonin, also known as 5-hydroxytryptamine, are believed to be involved.[3]
Pathophysiology
Aura
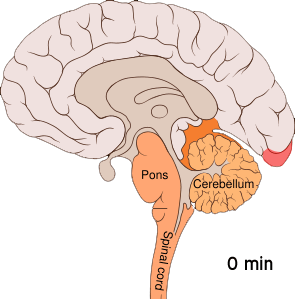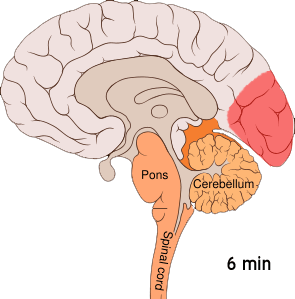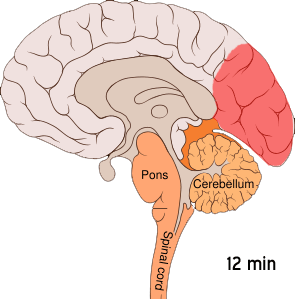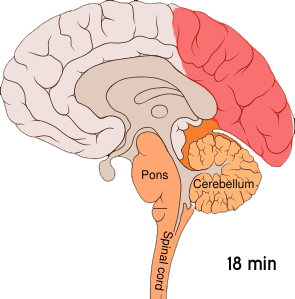
Cortical spreading depression or spreading depression of Leão is bursts of neuronal activity followed by a period of inactivity, which is seen in those with migraines with an aura.[7] There are a number of explanations for its occurrence including activation of NMDA receptors leading to calcium entering the cell.[7] After the burst of activity the blood flow to the cerebral cortex in the area affected is decreased for two to six hours.[7] It is believed that when depolarization travels down the underside of the brain, nerves that sense pain in the head and neck are triggered.[7]
Shown below is an image depicting cortical spreading depression which is the underlying pathophysiology of aura.
Pain
The exact mechanism of the head pain which occurs during a migraine is unknown.[8] Some evidence supports a primary role for central nervous system structures (such as the brainstem and diencephalon)[9] while other data support the role peripheral activation (such as via the sensory nerves that surround blood vessels of the head and neck).[8] The potential candidate vessels include dural arteries, pial arteries and extracranial arteries such as those of the scalp.[8] The role of vasodilatation of the extracranial arteries, in particular, is believed to be significant.[10]
Genetics
Studies of twins indicate a 34% to 51% genetic influence of likelihood to develop migraine headaches.[11] This genetic relationship is stronger for migraines with aura than for migraines without aura. A number of specific variants of genes increase the risk by a small to moderate amount.[12]
Single gene disorders that result in migraines are rare.[12] One of these is known as familial hemiplegic migraine, a type of migraine with aura, which is inherited in anautosomal dominant fashion.[13][14] Four genes have been shown to be involved in familial hemiplegic migraine.[15] Three of these genes are involved in ion transport.[15] The fourth is an axonal protein associated with the exocytosis complex.[15] Another genetic disorder associated with migraine is CADASIL syndrome or cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy.[16]
Evolution
Defense mechanism
The tendency to develop head pain when faced with a stressor or strong sensory stimuli can be explained in two ways. First, it may be a side effect of other CNS processes that provide important evolutionary advantages. One example is counteracting the dilation of cranial arteries to counteract dangerous vasoconstriction in the brain.[17] Second, migraine may be an example of how pain has evolved to encourage organisms to avoid potentially harmful situations. Olfactory-induced migraines (migraines stimulated by strong smells) have been explained as an attempt to interrupt the entry of toxins into the brain via the olfactory nerve.[18] Similarly, the low threshold for nausea and vomiting may be a mechanism to enhance elimination of ingested toxins in food. Migraineurs have a lower prevalence of malignant neoplasms in the brain than controls, suggesting that migraines are protective against tumors. However, the mechanism responsible for this difference is unknown.[19]
Conflicts with other organisms
A headache-prone CNS may have resulted from interactions with other organisms in two ways. The first possibility is that migraine offers an advantage to the organism in fighting infection by increasing blood flow to the brain. The second possibility is that certain pathogens evolved to cause headache as a way of speeding their transmission to other organisms.[20] Finally, migraine may benefit neither the host nor the pathogen, but may simply be the result of certain infections.[21] This last explanation is concordant with the apparent negative impact of migraine on human fitness.
Novel environmental factors
Modern environmental factors, with an increased sensory overload, may be especially permissive of the expression of genes that predispose for the disorder. If so, natural selection may not have had a chance to eliminate these genes yet. The increasing prevalence of migraine is easily a result of increased known triggers, such as bright light, loud noise, altered sleep/wake patterns, and emotional stress. This is an example of mismatch theory, which states that the current environment differs from the evolutionary environment of a particular trait.[20]
Genetic harms and benefits
Migraine is influenced on a polygenetic level (controlled by multiple genes). Therefore, researchers have theorized that migraine is a tradeoff and that it exists as a spectrum of susceptibility, with the majority of the population falling in the "heterozygous" zone between the two extremes of experiencing no headache and experiencing frequent, incapacitating headache. While it is not known for certain how or whether mild forms of the disorder would enhance survival, there is evidence of enhanced visual sensitivity in family members of migraineurs.[22] Additionally, this compromise theory may explain the higher prevalence among women, especially pregnant women and women of reproductive age (25-40). The avoidance of threatening environments is historically more important to the reproductive success of women.[23] The compromise between genetic harms and benefits is commonly seen in other disorders, such as cystic fibrosis and sickle cell anemia.
Headache as a design construct
Finally, migraine may be a component of imperfect central nervous system design. Evidence has suggested a dysfunction of pain-inhibitory pathways in migraine and discordant interaction between the ancient brain stem design and the more evolved neocortex.[24] The brain stem may be unable to suppress excessive input from higher brain centers.
References
- ↑ 1.0 1.1 Goadsby PJ, Lipton RB, Ferrari MD (2002). "Migraine--current understanding and treatment". N Engl J Med. 346 (4): 257–70. doi:10.1056/NEJMra010917. PMID 11807151.
- ↑ Bartleson JD, Cutrer FM (May 2010). "Migraine update. Diagnosis and treatment". Minn Med. 93 (5): 36–41. PMID 20572569.
- ↑ 3.0 3.1 The Headaches Chp. 29, Pg. 276
- ↑ Goadsby, PJ (January 2009). "The vascular theory of migraine – a great story wrecked by the facts". Brain : a journal of neurology. 132 (Pt 1): 6–7. doi:10.1093/brain/awn321. PMID 19098031.
- ↑ Brennan, KC (June 2010). "An update on the blood vessel in migraine". Current Opinion in Neurology. 23 (3): 266–74. doi:10.1097/WCO.0b013e32833821c1. PMID 20216215. Unknown parameter
|coauthors=ignored (help) - ↑ Dodick, DW (April 2008). "Examining the essence of migraine – is it the blood vessel or the brain? A debate". Headache. 48 (4): 661–7. doi:10.1111/j.1526-4610.2008.01079.x. PMID 18377395.
- ↑ 7.0 7.1 7.2 7.3 The Headaches, Chp. 28, pp. 269–72
- ↑ 8.0 8.1 8.2 Olesen, J (July 2009). "Origin of pain in migraine: evidence for peripheral sensitization". Lancet neurology. 8 (7): 679–90. doi:10.1016/S1474-4422(09)70090-0. PMID 19539239. Unknown parameter
|coauthors=ignored (help) - ↑ Akerman, S (2011-09-20). "Diencephalic and brainstem mechanisms in migraine". Nature Reviews Neuroscience. 12 (10): 570–84. doi:10.1038/nrn3057. PMID 21931334. Unknown parameter
|coauthors=ignored (help) - ↑ Shevel, E (March 2011). "The extracranial vascular theory of migraine – a great story confirmed by the facts". Headache. 51 (3): 409–17. doi:10.1111/j.1526-4610.2011.01844.x. PMID 21352215.
- ↑ Piane, M (December 2007). "Genetics of migraine and pharmacogenomics: some considerations". The journal of headache and pain (Review). 8 (6): 334–9. doi:10.1007/s10194-007-0427-2. PMC 2779399. PMID 18058067. Unknown parameter
|coauthors=ignored (help) - ↑ 12.0 12.1 Schürks, M (January 2012). "Genetics of migraine in the age of genome-wide association studies". The journal of headache and pain (Review). 13 (1): 1–9. doi:10.1007/s10194-011-0399-0. PMC 3253157. PMID 22072275.
- ↑ de Vries, B (July 2009). "Molecular genetics of migraine". Human Genetics (Review). 126 (1): 115–32. doi:10.1007/s00439-009-0684-z. PMID 19455354. Unknown parameter
|coauthors=ignored (help) - ↑ Montagna, P (September 2008). "Migraine genetics". Expert Review of Neurotherapeutics (Review). 8 (9): 1321–30. doi:10.1586/14737175.8.9.1321. PMID 18759544.
- ↑ 15.0 15.1 15.2 Ducros, A (Apr 22, 2013). "[Genetics of migraine]". Revue neurologique. 169 (5): 360–71. doi:10.1016/j.neurol.2012.11.010. PMID 23618705.
- ↑ Aminoff, Roger P. Simon, David A. Greenberg, Michael J. (2009). Clinical neurology (7 ed.). New York, N.Y: Lange Medical Books/McGraw-Hill. pp. 85–88. ISBN 9780071664332.
- ↑ [unreliable medical source?]Edvinsson, L. (1 October 1995). "Modification of vasoconstrictor responses in cerebral blood vessels by lesioning of the trigeminal nerve: possible involvement of CGRP". Cephalalgia. 15 (5): 373–383. doi:10.1046/j.1468-2982.1995.1505373.x. PMID 8536296. Unknown parameter
|coauthors=ignored (help) - ↑ [unreliable medical source?]Covelli, Vito (1 January 1994). "Could migraine be a "protective factor" against tumors?". International Journal of Neuroscience. 75 (1–2): 139–143. doi:10.3109/00207459408986297. PMID 8050847. Unknown parameter
|coauthors=ignored (help) - ↑ [unreliable medical source?]Snyder, RD (1 November 1997). "Olfaction in migraine". Cephalalgia (Clinical trial). 17 (7): 729–732. doi:10.1046/j.1468-2982.1997.1707729.x. PMID 9399001. Unknown parameter
|coauthors=ignored (help) - ↑ 20.0 20.1
- ↑ Cochran, Gregory M (1 January 2000). "Infectious causation of disease: an evolutionary perspective". Perspectives in Biology and Medicine (Historical article). 43 (3): 406–448. doi:10.1353/pbm.2000.0016. PMID 10893730. Unknown parameter
|coauthors=ignored (help) - ↑ [unreliable medical source?]Puca, F. (1 June 1996). "Photic driving in migraine: correlations with clinical features". Cephalalgia. 16 (4): 246–250. doi:10.1046/j.1468-2982.1996.1604246.x. PMID 8792036. Unknown parameter
|coauthors=ignored (help) - ↑ [unreliable medical source?]Stewart, Walter F. (1 January 1992). "Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors". JAMA: The Journal of the American Medical Association. 267 (1): 64. doi:10.1001/jama.1992.03480010072027. PMID 1727198.
- ↑ [unreliable medical source?]Weiller, C (July 1995). "Brain stem activation in spontaneous human migraine attacks". Nature medicine. 1 (7): 658–60. PMID 7585147. Unknown parameter
|coauthors=ignored (help)
- Pages with reference errors
- CS1 maint: Multiple names: authors list
- Pages with citations using unsupported parameters
- All articles lacking reliable references
- Articles lacking reliable references from December 2013
- Articles with invalid date parameter in template
- Needs overview
- Migraine
- Neurology
- Emergency medicine
- Disease
- Headaches
- Head and neck