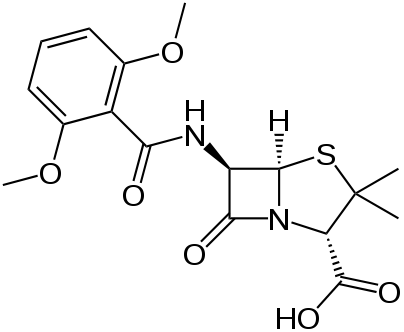
Methicillin
| 6-(2,6-dimethoxybenzamido)-3,3-dimethyl- 7-oxo-4-thia-1-azabicyclo[3.2.0]heptane- 2-carboxylic acid | |
| CAS number 61-32-5 |
ATC code J01CF03 |
| PubChem CID 6087 |
DrugBank n/a |
| Chemical formula | C17H20N2O6S |
| Molecular weight | 380.42 |
| Bioavailability | ? (not orally-absorbed) |
| Metabolism | hepatic, 20–40% |
| Elimination half-life | 25–60 minutes |
| Excretion | renal |
| Pregnancy category | ? |
| Legal status | ? |
| Routes of administration | IV |
|
WikiDoc Resources for Methicillin |
|
Articles |
|---|
|
Most recent articles on Methicillin Most cited articles on Methicillin |
|
Media |
|
Powerpoint slides on Methicillin |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Methicillin at Clinical Trials.gov Clinical Trials on Methicillin at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Methicillin
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Methicillin Discussion groups on Methicillin Patient Handouts on Methicillin Directions to Hospitals Treating Methicillin Risk calculators and risk factors for Methicillin
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Methicillin |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [1] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Methicillin (USAN) or meticillin (INN, BAN) is a narrow spectrum beta-lactam antibiotic of the penicillin class. It was developed by Beecham in 1959. It was previously used to treat infections caused by susceptible Gram-positive bacteria, particularly beta-lactamase-producing organisms such as Staphylococcus aureus that would otherwise be resistant to most penicillins, but is no longer clinically used. Its role in therapy has been largely replaced by flucloxacillin and dicloxacillin, however the term methicillin-resistant Staphylococcus aureus (MRSA) continues to be used to describe Staphylococcus aureus strains resistant to all penicillins.
Mode of action
Main article: Beta-lactam antibiotic
Like other beta-lactam antibiotics, methicillin acts by inhibiting the synthesis of bacterial cell walls. It inhibits cross-linkage between the linear peptidoglycan polymer chains that make up a major component of the cell wall of Gram-positive bacteria. It does this by binding to and competitively inhibiting the transpeptidase enzyme used by bacteria to cross-link the peptide (D-alanyl-alanine) used in peptidogylcan synthesis. Methicillin and other beta-lactam antibiotics are structural analogs of D-alanyl-alanine, and the transpeptidase enzymes that bind to them are sometimes called penicillin binding proteins (PBPs). (Gladwin and Trattler, 2004)
Medicinal chemistry
Methicillin is insensitive to beta-lactamase (also known as penicillinase) enzymes secreted by many penicillin-resistant bacteria. The presence of the ortho-dimethoxyphenyl group directly attached to the side chain carbonyl group of the penicillin nucleus facilitates the β-lactamase resistance, since those enzymes are relatively intolerant of side-chain steric hindrance. Thus it is able to bind to penicillin binding proteins (PBPs) and inhibit peptidoglycan crosslinking, but is not bound by or inactivated by β-lactamases.
Clinical use
Methicillin is not commonly used in clinical practice, but serves a purpose in the laboratory to determine antibiotic sensitivity in microbiological culture. Methicillin was previously used to treat infections caused by susceptible Gram-positive bacteria. It is unstable in the presence of gastric acid, with a degradation half-life of 5 minutes at pH 2, so it must be administered by injection. (Mitscher, 2002)
See also
References
- Mitscher LA. Antibiotics and antimicrobial agents. In: Williams DA, Lemke TL, editors. Foye's Principles of medicinal chemistry, 5th edition. Philadelphia: Lippincott Williams & Wilkins; 2002.
- Gladwin M., Trattler B. Clinical Microbiology made ridiculously simple. 3rd edition. Miami: MedMaster, Inc.; 2004.
Template:SIB de:Methicillin nl:Meticilline sr:Метицилин th:เมทิซิลลิน Template:WH Template:WikiDoc Sources