Methicillin: Difference between revisions
m (Protected "Methicillin": Protecting pages from unwanted edits ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
Kiran Singh (talk | contribs) |
||
| (5 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{| | {{drugbox | ||
| | | Verifiedfields = changed | ||
| | | Watchedfields = changed | ||
| verifiedrevid = 404016571 | |||
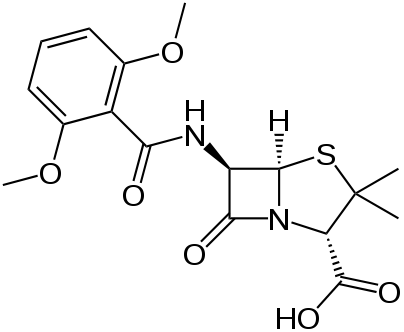
'' | | IUPAC_name = (2''S'',5''R'',6''R'')-6-(2,6-dimethoxybenzamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid | ||
| image =Methicillin.png | |||
|- | <!--Clinical data--> | ||
| | | pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | ||
| | | pregnancy_US = <!-- A / B / C / D / X --> | ||
|- | | legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> | ||
| | | legal_CA = <!-- / Schedule I, II, III, IV, V, VI, VII, VIII --> | ||
| | | legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> | ||
| legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> | |||
| | | routes_of_administration = [[Intravenous|IV]] | ||
| | <!--Pharmacokinetic data--> | ||
| | | bioavailability = Not orally absorbed | ||
| | | metabolism = hepatic, 20–40% | ||
| elimination_half-life = 25–60 minutes | |||
| | | excretion = renal | ||
| | |||
|- | <!--Identifiers--> | ||
| | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| | | CAS_number_Ref = {{cascite|correct|??}} | ||
| | | CAS_number = 61-32-5 | ||
| | | ATC_prefix = J01 | ||
| | | ATC_suffix = CF03 | ||
| | | ATC_supplemental = {{ATCvet|J51|CF03}} | ||
| | | PubChem = 6087 | ||
| | | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | ||
| | | DrugBank = DB01603 | ||
| | | ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} | ||
| | | ChemSpiderID = 5862 | ||
| UNII_Ref = {{fdacite|changed|FDA}} | |||
| | | UNII = Q91FH1328A | ||
| | | ChEMBL_Ref = {{ebicite|changed|EBI}} | ||
| | | ChEMBL = 575 | ||
| | |||
<!--Chemical data--> | |||
| C=17 | H=20 | N=2 | O=6 | S=1 | |||
| molecular_weight = 380.42 g/mol | |||
| smiles = OC(=O)[C@@H]2N3C(=O)[C@@H](NC(=O)c1c(OC)cccc1OC)[C@H]3SC2(C)C | |||
}} | |||
__Notoc__ | |||
{{SI}} | {{SI}} | ||
{{ | {{CMG}} | ||
==Overview== | |||
'''Meticillin''' ([[International Nonproprietary Name|INN]], [[British Approved Name|BAN]]) or '''methicillin''' ([[United States Adopted Name|USAN]]) is a [[narrow-spectrum antibiotic|narrow-spectrum]] [[β-lactam antibiotic]] of the [[penicillin]] class. It should not be confused with the antibiotic [[metacycline]]. In 2005, the name of the drug was changed from methicillin to meticillin in accordance with the International Pharmacopoeia guidelines.<ref> UK parliament [http://www.publications.parliament.uk/pa/cm200405/cmhansrd/vo050405/text/50405w39.htm MRSA]</ref> | |||
==History== | |||
Meticillin was developed by [[Beecham (pharmaceutical company)|Beecham]] in 1959.<ref name="Dutfield2009">{{cite book|author=Graham Dutfield|title=Intellectual property rights and the life science industries: past, present and future|url=http://books.google.com/books?id=hnleY38aUxYC&pg=PA140|accessdate=18 November 2010|date=30 July 2009|publisher=World Scientific|isbn=978-981-283-227-6|pages=140–}}</ref> It was previously used to treat [[infection]]s caused by susceptible [[Gram-positive]] [[bacteria]], in particular, [[penicillinase]]-producing organisms such as ''[[Staphylococcus aureus]]'' that would otherwise be resistant to most penicillins. | |||
Its role in therapy has been largely replaced by [[flucloxacillin]] and [[dicloxacillin]], but the term [[methicillin-resistant Staphylococcus aureus|meticillin-resistant ''Staphylococcus aureus'']] (MRSA) continues to be used to describe ''S. aureus'' strains resistant to all penicillins.<ref>[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1079642/ MRSA—past, present, future]</ref> | |||
Meticillin is no longer manufactured because the more stable and similar penicillins such as [[oxacillin]] (used for clinical antimicrobial susceptibility testing), flucloxacillin, and dicloxacillin are used medically. | |||
== Mode of action == | |||
Like other beta-lactam antibiotics, meticillin acts by inhibiting the synthesis of bacterial [[cell wall]]s. It inhibits cross-linkage between the linear [[peptidoglycan]] polymer chains that make up a major component of the cell wall of Gram-positive bacteria. It does this by binding to and competitively inhibiting the [[DD-transpeptidase|transpeptidase]] enzyme (also known as [[penicillin-binding proteins]] (PBPs)). These PBPs crosslink glycopeptides (''<small>D</small>-alanyl-alanine''), forming the peptidoglycan cell wall. Meticillin and other β-lactam antibiotics are structural analogs of <small>D</small>-alanyl-alanine, and the transpeptidase enzymes that bind to them are sometimes called [[penicillin-binding proteins]] (PBPs).<ref>Gladwin M., Trattler B. Clinical Microbiology made ridiculously simple. 3rd edition. Miami: MedMaster, Inc.; 2004.</ref> | |||
Meticillin is actually a [[penicillinase]]-resistant B-lactam antibiotic. Penicillinase is a bacterial enzyme produced by bacteria resistant to other B-lactam antibiotics which hydrolyses the antibiotic, rendering it nonfunctional. Meticillin is not bound and hydrolysed by penicillinase, meaning it can kill the bacteria, even if this enzyme is present. | |||
== Spectrum of bacterial resistance and susceptibility == | |||
At one time, meticillin was used to treat infections caused by certain Gram-positive bacteria including ''Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pyogenes'', and ''Streptococcus pneumoniae''. Today, meticillin is not as effective against these organisms due to resistance. | |||
'' | Resistance to meticillin is conferred by activation of a new bacterial PBP gene (''[[mec2]]''). This encodes for the [[PBP2a]]. PBP2a works in a similar manner to other PBPs, but it is bound by β-lactams with very low affinity, meaning they cannot hydrolyse it and kill the bacteria. Expression of ''PBPA2'' confers resistance to all β-lactams. | ||
These susceptibility data are given on a few medically significant bacteria: | |||
'' | * ''Staphylococcus aureus'' - 0.125 - >100 μg/ml | ||
* Meticillin resistant ''Staphylococcus aureus'' ([[MRSA]]) - 15.6 - >1000 μg/ml | |||
* ''Streptococcus pneumoniae'' 0.39 μg/ml | |||
== Medicinal chemistry == | |||
Meticillin is insensitive to [[beta-lactamase]] (also known as penicillinase) enzymes secreted by many penicillin-resistant bacteria. The presence of the ''ortho''-dimethoxyphenyl group directly attached to the [[Side chain|side-chain]] carbonyl group of the penicillin nucleus facilitates the β-lactamase resistance, since those enzymes are relatively intolerant of side-chain [[steric hindrance]]. Thus, it is able to bind to PBPs and inhibit [[peptidoglycan]] crosslinking, but it is not bound by or inactivated by β-lactamases. | |||
==Clinical use== | == Clinical use == | ||
Meticillin is no longer used to treat patients. Compared to other β-lactamase-resistant penicillins, it is less active, can be administered only [[Route of administration#Parenteral|parenterally]], and has a higher frequency of [[interstitial nephritis]], an otherwise-rare side effect of penicillins. However, selection of Meticillin depended on the outcome of susceptibility test of the microorganism, since it is no longer produced, it is also not routinely tested any more. It also served a purpose in the [[laboratory]] to determine the antibiotic sensitivity of ''Staphylococcus aureus'' to other β-lactamase-resistant penicillins; this role has now been passed on to other penicillines, namely ''[[Cloxacillin]]'' as well as genetic testing for the presence of ''[[mecA]]'' gene by ''[[PCR]]''. | |||
==References== | == References == | ||
{{wikinews|Supergerm deaths soar, surpass AIDS in the United States}} | |||
{{reflist|2}} | |||
[[Category:Drug]] | |||
[[Category:Beta-lactam antibiotics]] | [[Category:Beta-lactam antibiotics]] | ||
[[Category:Phenol ethers]] | |||
[[ | |||
Latest revision as of 13:56, 10 April 2015
 | |
| Clinical data | |
|---|---|
| Routes of administration | IV |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | Not orally absorbed |
| Metabolism | hepatic, 20–40% |
| Elimination half-life | 25–60 minutes |
| Excretion | renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C17H20N2O6S |
| Molar mass | 380.42 g/mol |
| 3D model (JSmol) | |
| |
| | |
|
WikiDoc Resources for Methicillin |
|
Articles |
|---|
|
Most recent articles on Methicillin Most cited articles on Methicillin |
|
Media |
|
Powerpoint slides on Methicillin |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Methicillin at Clinical Trials.gov Clinical Trials on Methicillin at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Methicillin
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Methicillin Discussion groups on Methicillin Patient Handouts on Methicillin Directions to Hospitals Treating Methicillin Risk calculators and risk factors for Methicillin
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Methicillin |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Meticillin (INN, BAN) or methicillin (USAN) is a narrow-spectrum β-lactam antibiotic of the penicillin class. It should not be confused with the antibiotic metacycline. In 2005, the name of the drug was changed from methicillin to meticillin in accordance with the International Pharmacopoeia guidelines.[1]
History
Meticillin was developed by Beecham in 1959.[2] It was previously used to treat infections caused by susceptible Gram-positive bacteria, in particular, penicillinase-producing organisms such as Staphylococcus aureus that would otherwise be resistant to most penicillins.
Its role in therapy has been largely replaced by flucloxacillin and dicloxacillin, but the term meticillin-resistant Staphylococcus aureus (MRSA) continues to be used to describe S. aureus strains resistant to all penicillins.[3]
Meticillin is no longer manufactured because the more stable and similar penicillins such as oxacillin (used for clinical antimicrobial susceptibility testing), flucloxacillin, and dicloxacillin are used medically.
Mode of action
Like other beta-lactam antibiotics, meticillin acts by inhibiting the synthesis of bacterial cell walls. It inhibits cross-linkage between the linear peptidoglycan polymer chains that make up a major component of the cell wall of Gram-positive bacteria. It does this by binding to and competitively inhibiting the transpeptidase enzyme (also known as penicillin-binding proteins (PBPs)). These PBPs crosslink glycopeptides (D-alanyl-alanine), forming the peptidoglycan cell wall. Meticillin and other β-lactam antibiotics are structural analogs of D-alanyl-alanine, and the transpeptidase enzymes that bind to them are sometimes called penicillin-binding proteins (PBPs).[4]
Meticillin is actually a penicillinase-resistant B-lactam antibiotic. Penicillinase is a bacterial enzyme produced by bacteria resistant to other B-lactam antibiotics which hydrolyses the antibiotic, rendering it nonfunctional. Meticillin is not bound and hydrolysed by penicillinase, meaning it can kill the bacteria, even if this enzyme is present.
Spectrum of bacterial resistance and susceptibility
At one time, meticillin was used to treat infections caused by certain Gram-positive bacteria including Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pyogenes, and Streptococcus pneumoniae. Today, meticillin is not as effective against these organisms due to resistance.
Resistance to meticillin is conferred by activation of a new bacterial PBP gene (mec2). This encodes for the PBP2a. PBP2a works in a similar manner to other PBPs, but it is bound by β-lactams with very low affinity, meaning they cannot hydrolyse it and kill the bacteria. Expression of PBPA2 confers resistance to all β-lactams.
These susceptibility data are given on a few medically significant bacteria:
- Staphylococcus aureus - 0.125 - >100 μg/ml
- Meticillin resistant Staphylococcus aureus (MRSA) - 15.6 - >1000 μg/ml
- Streptococcus pneumoniae 0.39 μg/ml
Medicinal chemistry
Meticillin is insensitive to beta-lactamase (also known as penicillinase) enzymes secreted by many penicillin-resistant bacteria. The presence of the ortho-dimethoxyphenyl group directly attached to the side-chain carbonyl group of the penicillin nucleus facilitates the β-lactamase resistance, since those enzymes are relatively intolerant of side-chain steric hindrance. Thus, it is able to bind to PBPs and inhibit peptidoglycan crosslinking, but it is not bound by or inactivated by β-lactamases.
Clinical use
Meticillin is no longer used to treat patients. Compared to other β-lactamase-resistant penicillins, it is less active, can be administered only parenterally, and has a higher frequency of interstitial nephritis, an otherwise-rare side effect of penicillins. However, selection of Meticillin depended on the outcome of susceptibility test of the microorganism, since it is no longer produced, it is also not routinely tested any more. It also served a purpose in the laboratory to determine the antibiotic sensitivity of Staphylococcus aureus to other β-lactamase-resistant penicillins; this role has now been passed on to other penicillines, namely Cloxacillin as well as genetic testing for the presence of mecA gene by PCR.
References
| Wikinews has related news: Supergerm deaths soar, surpass AIDS in the United States |
- ↑ UK parliament MRSA
- ↑ Graham Dutfield (30 July 2009). Intellectual property rights and the life science industries: past, present and future. World Scientific. pp. 140–. ISBN 978-981-283-227-6. Retrieved 18 November 2010.
- ↑ MRSA—past, present, future
- ↑ Gladwin M., Trattler B. Clinical Microbiology made ridiculously simple. 3rd edition. Miami: MedMaster, Inc.; 2004.
- Pages with script errors
- Articles with changed ChemSpider identifier
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without KEGG source
- Articles without InChI source
- Drugs with no legal status
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Drug
- Beta-lactam antibiotics
- Phenol ethers