Mafenide: Difference between revisions
No edit summary |
|||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | |||
{{ | |authorTag= | ||
{{VP}} | |||
<!--Overview--> | |||
= | |genericName= | ||
= | |aOrAn= | ||
a | |||
[[Category: | |drugClass= | ||
[[Category: | |||
|indication= | |||
|hasBlackBoxWarning= | |||
Yes | |||
|adverseReactions= | |||
<!--Black Box Warning--> | |||
|blackBoxWarningTitle= | |||
Title | |||
|blackBoxWarningBody= | |||
<i><span style="color:#FF0000;">ConditionName: </span></i> | |||
* Content | |||
<!--Adult Indications and Dosage--> | |||
<!--FDA-Labeled Indications and Dosage (Adult)--> | |||
|fdaLIADAdult= | |||
=====Condition1===== | |||
*Mafenide Acetate,USP For 5% Topical Solution is indicated for use as an adjunctive topical antimicrobial agent to control bacterial infection when used under moist dressings over meshed autografts on excised burn wounds. | |||
<!--Off-Label Use and Dosage (Adult)--> | |||
<!--Guideline-Supported Use (Adult)--> | |||
|offLabelAdultGuideSupport= | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Non–Guideline-Supported Use (Adult)--> | |||
|offLabelAdultNoGuideSupport= | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Pediatric Indications and Dosage--> | |||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | |||
|fdaLIADPed= | |||
=====Condition1===== | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Off-Label Use and Dosage (Pediatric)--> | |||
<!--Guideline-Supported Use (Pediatric)--> | |||
|offLabelPedGuideSupport= | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Non–Guideline-Supported Use (Pediatric)--> | |||
|offLabelPedNoGuideSupport= | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | |||
|contraindications= | |||
* Mafenide Acetate ,USP For 5% Topical Solution is contraindicated in patients who are [[hypersensitive]] to mafenide acetate. It is not known whether there is cross sensitivity to other [[sulfonamides]]. | |||
<!--Warnings--> | |||
|warnings= | |||
* Fatal [[hemolytic anemia]] with disseminated intravascular [[coagulation]], presumably related to a [[glucose-6-phosphate dehydrogenase deficiency]], has been reported following therapy with Mafenide Acetate. | |||
====Precautions==== | |||
* Mafenide Acetate and its metabolite, p-carboxybenzenesulfonamide, inhibit [[carbonic anhydrase]], which may result in [[metabolic acidosis]], usually compensated by [[hyperventilation]]. In the presence of impaired [[renal function]], high blood levels of Mafenide Acetate and its metabolite may exaggerate the carbonic anhydrase inhibition. Therefore, close monitoring of acid-base balance is necessary, particularly in patients with extensive second-degree or partial thickness [[burns]] and in those with pulmonary or renal dysfunction. Some burn patients treated with Mafenide Acetate have also been reported to manifest an unexplained syndrome of masked [[hyperventilation]] with resulting [[respiratory alkalosis]] (slightly alkaline blood [[pH]], low arterial pCO2, and decreased total CO2); change in arterial pO2 is variable. The etiology and significance of these findings are unknown. | |||
*Mafenide Acetate should be used with caution in burn patients with [[acute renal failure]]. | |||
*[[Fungal]] colonization may occur concomitantly with reduction of bacterial growth in the burn wound. However, systemic [[fungal infection]] through the infected [[burn]] wound is rare. | |||
<!--Adverse Reactions--> | |||
<!--Clinical Trials Experience--> | |||
|clinicalTrials= | |||
*In the clinical setting of severe burns, it is often difficult to distinguish between an adverse reaction to Mafenide Acetate and burn sequelae. In a clinical study of pediatric patients with acute burns requiring autografts who received Mafenide Acetate,USP for 5% SOLUTION in addition to double antibiotic solution (DAB) wound therapy (neomycin sulfate 40 mg and polymyxin B 200,000 units/ liter), the incidence of rash (4.6%) and itching (2.8%) in the group which received Mafenide Acetate USP For 5% Solution was not different from that experienced with (DAB) dressings alone (5.7% and 1.3%, respectively). | |||
*From other clinical settings, a single case of bone marrow depression and a single case of an acute attack of porphyria have been reported following therapy with Mafenide Acetate. Fatal hemolytic anemia with disseminated intravascular coagulation, presumably related to a glucose-6-phosphate dehydrogenase deficiency, has been reported following therapy with mafenide acetate. The following adverse reactions have been reported with topical Mafenide Acetate therapy: | |||
=====Dermatologic and Allergic===== | |||
Pain or burning sensation, rash and pruritus (often localized to the area covered by the wound dressing), erythema, skin maceration from prolonged wet dressings, facial edema, swelling, hives, blisters, eosinophilia. | |||
=====Respiratory or Metabolic===== | |||
Tachypnea, hyperventilation, decrease in pCO 2, metabolic acidosis, increase in serum chloride. | |||
<!--Postmarketing Experience--> | |||
|postmarketing= | |||
There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |||
<!--Drug Interactions--> | |||
|drugInteractions= | |||
<!--Use in Specific Populations--> | |||
|useInPregnancyFDA= | |||
* '''Pregnancy Category C''' | |||
*A teratology study performed in rats using oral doses of up to 600 mg/kg/day revealed no evidence of harm to the fetus due to Mafenide Acetate. There are no adequate data regarding the potential reproductive toxicity of Mafenide Acetate in a non-rodent species, nor are there adequate and well-controlled studies in pregnant women. Mafenide Acetate should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. | |||
|useInPregnancyAUS= | |||
* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | |||
|useInLaborDelivery= | |||
There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |||
|useInNursing= | |||
*It is not known whether Mafenide Acetate is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from Mafenide Acetate, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. | |||
|useInPed= | |||
*The safety and effectiveness of Mafenide Acetate,USP For 5% Topical Solution have been established in the age groups 3 months to 16 years. | |||
|useInGeri= | |||
*No studies have been conducted to specifically examine the effects of Mafenide Acetate on burn wounds in geriatric patients. | |||
|useInGender= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |||
|useInRace= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |||
|useInHepaticImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | |||
|useInReproPotential= | |||
There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInImmunocomp= | |||
There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
<!--Administration and Monitoring--> | |||
|administration= | |||
* Topical | |||
|monitoring= | |||
There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |||
<!--IV Compatibility--> | |||
|IVCompat= | |||
There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
<!--Overdosage--> | |||
|overdose= | |||
===Acute Overdose=== | |||
*Single oral doses of 2000 mg/kg of Mafenide Acetate as a 5% solution did not cause mortality or clinical symptoms of toxicity in rats. | |||
===Chronic Overdose=== | |||
There is limited information regarding <i>Chronic Overdose</i> of {{PAGENAME}} in the drug label. | |||
<!--Pharmacology--> | |||
<!--Drug box 2--> | |||
|drugBox= | |||
{{Drugbox2 | |||
| Verifiedfields = changed | |||
| Watchedfields = changed | |||
| verifiedrevid = 462096004 | |||
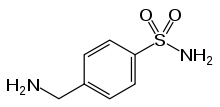
| IUPAC_name = 4-(Aminomethyl)benzenesulfonamide | |||
| image = Mafenide00.png | |||
| image2 = Mafenide000.png | |||
<!--Clinical data--> | |||
| tradename = | |||
| Drugs.com = {{drugs.com|monograph|sulfamylon}} | |||
| pregnancy_category = | |||
| legal_status = | |||
| routes_of_administration = Topical | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| metabolism = | |||
| excretion = | |||
<!--Identifiers--> | |||
| CAS_number_Ref = {{cascite|changed|??}} | |||
| CAS_number = 138-39-6 | |||
| ATC_prefix = D06 | |||
| ATC_suffix = BA03 | |||
| PubChem = 3998 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 3858 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 58447S8P4L | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D02351 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 419 | |||
<!--Chemical data--> | |||
| C=7 | H=10 | N=2 | O=2 | S=1 | |||
| molecular_weight = 186.233 g/mol | |||
| smiles = O=S(=O)(c1ccc(cc1)CN)N | |||
| InChI = 1/C7H10N2O2S/c8-5-6-1-3-7(4-2-6)12(9,10)11/h1-4H,5,8H2,(H2,9,10,11) | |||
| InChIKey = TYMRLRRVMHJFTF-UHFFFAOYAA | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C7H10N2O2S/c8-5-6-1-3-7(4-2-6)12(9,10)11/h1-4H,5,8H2,(H2,9,10,11) | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = TYMRLRRVMHJFTF-UHFFFAOYSA-N | |||
}} | |||
<!--Mechanism of Action--> | |||
|mechAction= | |||
* The mechanism of action of Mafenide is not known, but is different from that of the [[sulfonamides]]. Mafenide is not antagonized by pABA, serum, pus or tissue exudates, and there is no correlation between bacterial sensitivities to mafenide and to the [[sulfonamides]]. Its activity is not altered by changes in the acidity of the environment. The [[osmolality]] of the 5% topical solution is approximately 340 mOsm/kg. | |||
<!--Structure--> | |||
|structure= | |||
* Mafenide Acetate, USP is a synthetic antimicrobial agent designated chemically as α-amino- p-toluenesulfonamide monoacetate. It has the following structural formula: | |||
: [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
*Mafenide Acetate, USP is a white, crystalline powder which is freely soluble in water. | |||
*Mafenide Acetate,USP For 5% Topical Solution is provided in packets containing 50 g of sterile Mafenide Acetate to be reconstituted in 1000 mL of Sterile Water for Irrigation, USP or 0.9% Sodium Chloride Irrigation, USP. After mixing, the solution contains 5% w/v of mafenide acetate. The solution is an antimicrobial preparation suitable for topical administration. The solution is not for injection. The reconstituted solution may be held up to 28 days after preparation if stored in unopened containers. ONCE A CONTAINER IS OPENED, ANY UNUSED PORTION SHOULD BE DISCARDED AFTER 48 HOURS. Store the reconstituted solution at 20° to 25°C (68° to 77°F). Limited storage periods at 15° to 30°C (59° to 86°F) are acceptable. | |||
<!--Pharmacodynamics--> | |||
|PD= | |||
There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |||
<!--Pharmacokinetics--> | |||
|PK= | |||
*Applied topically, Mafenide Acetate diffuses through devascularized areas. Approximately 80% of a Mafenide Acetate dose is delivered to burned tissue over four hours following topical application of the 5% solution. Following application of Mafenide Acetate cream and solution, peak Mafenide concentrations in human burned skin tissue occur at two and four hours, respectively. Peak tissue concentrations are similar following administration of the solution or cream. Once absorbed, Mafenide is rapidly converted to an inactive metabolite (p-carboxybenzenesulfonamide) which is cleared through the kidneys. Clinical studies have shown that when applied topically to burns as an 11.2% Mafenide Acetate cream, blood levels of the parent drug peaked at 2 hours following application, ranging from 26 to 197 µg/mL for single doses of 14 to 77 g of Mafenide Acetate. Metabolite levels peaked at 3 hours, ranging from 10 to 340 µg/mL. Twenty-four hours after application, combined parent and metabolite blood levels had fallen to pretreatment levels. | |||
<!--Nonclinical Toxicology--> | |||
|nonClinToxic= | |||
*No long-term animal studies have been performed to evaluate the carcinogenic potential of Mafenide Acetate; however, the drug did not induce mutations in L5178Y mouse lymphoma cells at the TK locus. | |||
*Animal studies have not been performed to evaluate the potential effects of Mafenide Acetate on fertility. | |||
<!--Clinical Studies--> | |||
|clinicalStudies= | |||
There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | |||
<!--How Supplied--> | |||
|howSupplied= | |||
* Mafenide Acetate , USP for 5% Topical Solution is available in packets containing 50 g of sterile Mafenide Acetate to be prepared using 1000 mL Sterile Water for Irrigation, USP or 0.9% Sodium Chloride Irrigation, USP. (See DOSAGE AND ADMINISTRATION: MAFENIDE ACETATE USP for 5% Topical Solution: Directions for Preparation of the Solution.) The packets are supplied as follows: | |||
:*Carton of five 50 g packets (NDC # 49884-902-78). | |||
*Recommended Storage | |||
:*Packets - Store PACKETS in a dry place at room temperature 15° to 30°C (59° to 86°F). | |||
:*Prepared Solution - Store SOLUTION at 20° to 25°C (68° to 77°F) with excursions permitted to 15° to 30°C (59° to 86°F). | |||
:*The solution may be held for up to 28 days if stored in unopened containers. | |||
:*ONCE A CONTAINER IS OPENED, ANY UNUSED SOLUTION MUST BE DISCARDED WITHIN 48 HOURS. | |||
<!--Patient Counseling Information--> | |||
|fdaPatientInfo= | |||
There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | |||
<!--Precautions with Alcohol--> | |||
|alcohol= | |||
* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
<!--Brand Names--> | |||
|brandNames= | |||
* MAFENIDE ACETATE®<ref>{{Cite web | title = MAFENIDE ACETATE mafenide acetate powder, for solution | url = http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e48cc935-2058-48c0-9281-b8b919629493 }}</ref> | |||
<!--Look-Alike Drug Names--> | |||
|lookAlike= | |||
<!--Drug Shortage Status--> | |||
|drugShortage= | |||
}} | |||
<!--Pill Image--> | |||
{{PillImage | |||
|fileName=No image.jpg|This image is provided by the National Library of Medicine. | |||
|drugName= | |||
|NDC= | |||
|drugAuthor= | |||
|ingredients= | |||
|pillImprint= | |||
|dosageValue= | |||
|dosageUnit= | |||
|pillColor= | |||
|pillShape= | |||
|pillSize= | |||
|pillScore= | |||
}} | |||
<!--Label Display Image--> | |||
{{LabelImage | |||
|fileName={{PAGENAME}}11.png|This image is provided by the National Library of Medicine. | |||
}} | |||
{{LabelImage | |||
|fileName={{PAGENAME}}11.png|This image is provided by the National Library of Medicine. | |||
}} | |||
<!--Category--> | |||
[[Category:Drug]] | |||
Revision as of 18:29, 17 February 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Title
See full prescribing information for complete Boxed Warning.
ConditionName:
|
Overview
Mafenide is a that is FDA approved for the {{{indicationType}}} of . There is a Black Box Warning for this drug as shown here. Common adverse reactions include .
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition1
- Mafenide Acetate,USP For 5% Topical Solution is indicated for use as an adjunctive topical antimicrobial agent to control bacterial infection when used under moist dressings over meshed autografts on excised burn wounds.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Mafenide in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Mafenide in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Mafenide in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Mafenide in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Mafenide in pediatric patients.
Contraindications
- Mafenide Acetate ,USP For 5% Topical Solution is contraindicated in patients who are hypersensitive to mafenide acetate. It is not known whether there is cross sensitivity to other sulfonamides.
Warnings
|
Title
See full prescribing information for complete Boxed Warning.
ConditionName:
|
- Fatal hemolytic anemia with disseminated intravascular coagulation, presumably related to a glucose-6-phosphate dehydrogenase deficiency, has been reported following therapy with Mafenide Acetate.
Precautions
- Mafenide Acetate and its metabolite, p-carboxybenzenesulfonamide, inhibit carbonic anhydrase, which may result in metabolic acidosis, usually compensated by hyperventilation. In the presence of impaired renal function, high blood levels of Mafenide Acetate and its metabolite may exaggerate the carbonic anhydrase inhibition. Therefore, close monitoring of acid-base balance is necessary, particularly in patients with extensive second-degree or partial thickness burns and in those with pulmonary or renal dysfunction. Some burn patients treated with Mafenide Acetate have also been reported to manifest an unexplained syndrome of masked hyperventilation with resulting respiratory alkalosis (slightly alkaline blood pH, low arterial pCO2, and decreased total CO2); change in arterial pO2 is variable. The etiology and significance of these findings are unknown.
- Mafenide Acetate should be used with caution in burn patients with acute renal failure.
- Fungal colonization may occur concomitantly with reduction of bacterial growth in the burn wound. However, systemic fungal infection through the infected burn wound is rare.
Adverse Reactions
Clinical Trials Experience
- In the clinical setting of severe burns, it is often difficult to distinguish between an adverse reaction to Mafenide Acetate and burn sequelae. In a clinical study of pediatric patients with acute burns requiring autografts who received Mafenide Acetate,USP for 5% SOLUTION in addition to double antibiotic solution (DAB) wound therapy (neomycin sulfate 40 mg and polymyxin B 200,000 units/ liter), the incidence of rash (4.6%) and itching (2.8%) in the group which received Mafenide Acetate USP For 5% Solution was not different from that experienced with (DAB) dressings alone (5.7% and 1.3%, respectively).
- From other clinical settings, a single case of bone marrow depression and a single case of an acute attack of porphyria have been reported following therapy with Mafenide Acetate. Fatal hemolytic anemia with disseminated intravascular coagulation, presumably related to a glucose-6-phosphate dehydrogenase deficiency, has been reported following therapy with mafenide acetate. The following adverse reactions have been reported with topical Mafenide Acetate therapy:
Dermatologic and Allergic
Pain or burning sensation, rash and pruritus (often localized to the area covered by the wound dressing), erythema, skin maceration from prolonged wet dressings, facial edema, swelling, hives, blisters, eosinophilia.
Respiratory or Metabolic
Tachypnea, hyperventilation, decrease in pCO 2, metabolic acidosis, increase in serum chloride.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Mafenide in the drug label.
Drug Interactions
There is limited information regarding Mafenide Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Pregnancy Category C
- A teratology study performed in rats using oral doses of up to 600 mg/kg/day revealed no evidence of harm to the fetus due to Mafenide Acetate. There are no adequate data regarding the potential reproductive toxicity of Mafenide Acetate in a non-rodent species, nor are there adequate and well-controlled studies in pregnant women. Mafenide Acetate should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Mafenide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Mafenide during labor and delivery.
Nursing Mothers
- It is not known whether Mafenide Acetate is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from Mafenide Acetate, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- The safety and effectiveness of Mafenide Acetate,USP For 5% Topical Solution have been established in the age groups 3 months to 16 years.
Geriatic Use
- No studies have been conducted to specifically examine the effects of Mafenide Acetate on burn wounds in geriatric patients.
Gender
There is no FDA guidance on the use of Mafenide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Mafenide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Mafenide in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Mafenide in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Mafenide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Mafenide in patients who are immunocompromised.
Administration and Monitoring
Administration
- Topical
Monitoring
There is limited information regarding Monitoring of Mafenide in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Mafenide in the drug label.
Overdosage
Acute Overdose
- Single oral doses of 2000 mg/kg of Mafenide Acetate as a 5% solution did not cause mortality or clinical symptoms of toxicity in rats.
Chronic Overdose
There is limited information regarding Chronic Overdose of Mafenide in the drug label.
Pharmacology
| |
| |
Mafenide
| |
| Systematic (IUPAC) name | |
| 4-(Aminomethyl)benzenesulfonamide | |
| Identifiers | |
| CAS number | |
| ATC code | D06 |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 186.233 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | Topical |
Mechanism of Action
- The mechanism of action of Mafenide is not known, but is different from that of the sulfonamides. Mafenide is not antagonized by pABA, serum, pus or tissue exudates, and there is no correlation between bacterial sensitivities to mafenide and to the sulfonamides. Its activity is not altered by changes in the acidity of the environment. The osmolality of the 5% topical solution is approximately 340 mOsm/kg.
Structure
- Mafenide Acetate, USP is a synthetic antimicrobial agent designated chemically as α-amino- p-toluenesulfonamide monoacetate. It has the following structural formula:
- Mafenide Acetate, USP is a white, crystalline powder which is freely soluble in water.
- Mafenide Acetate,USP For 5% Topical Solution is provided in packets containing 50 g of sterile Mafenide Acetate to be reconstituted in 1000 mL of Sterile Water for Irrigation, USP or 0.9% Sodium Chloride Irrigation, USP. After mixing, the solution contains 5% w/v of mafenide acetate. The solution is an antimicrobial preparation suitable for topical administration. The solution is not for injection. The reconstituted solution may be held up to 28 days after preparation if stored in unopened containers. ONCE A CONTAINER IS OPENED, ANY UNUSED PORTION SHOULD BE DISCARDED AFTER 48 HOURS. Store the reconstituted solution at 20° to 25°C (68° to 77°F). Limited storage periods at 15° to 30°C (59° to 86°F) are acceptable.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Mafenide in the drug label.
Pharmacokinetics
- Applied topically, Mafenide Acetate diffuses through devascularized areas. Approximately 80% of a Mafenide Acetate dose is delivered to burned tissue over four hours following topical application of the 5% solution. Following application of Mafenide Acetate cream and solution, peak Mafenide concentrations in human burned skin tissue occur at two and four hours, respectively. Peak tissue concentrations are similar following administration of the solution or cream. Once absorbed, Mafenide is rapidly converted to an inactive metabolite (p-carboxybenzenesulfonamide) which is cleared through the kidneys. Clinical studies have shown that when applied topically to burns as an 11.2% Mafenide Acetate cream, blood levels of the parent drug peaked at 2 hours following application, ranging from 26 to 197 µg/mL for single doses of 14 to 77 g of Mafenide Acetate. Metabolite levels peaked at 3 hours, ranging from 10 to 340 µg/mL. Twenty-four hours after application, combined parent and metabolite blood levels had fallen to pretreatment levels.
Nonclinical Toxicology
- No long-term animal studies have been performed to evaluate the carcinogenic potential of Mafenide Acetate; however, the drug did not induce mutations in L5178Y mouse lymphoma cells at the TK locus.
- Animal studies have not been performed to evaluate the potential effects of Mafenide Acetate on fertility.
Clinical Studies
There is limited information regarding Clinical Studies of Mafenide in the drug label.
How Supplied
- Mafenide Acetate , USP for 5% Topical Solution is available in packets containing 50 g of sterile Mafenide Acetate to be prepared using 1000 mL Sterile Water for Irrigation, USP or 0.9% Sodium Chloride Irrigation, USP. (See DOSAGE AND ADMINISTRATION: MAFENIDE ACETATE USP for 5% Topical Solution: Directions for Preparation of the Solution.) The packets are supplied as follows:
- Carton of five 50 g packets (NDC # 49884-902-78).
- Recommended Storage
- Packets - Store PACKETS in a dry place at room temperature 15° to 30°C (59° to 86°F).
- Prepared Solution - Store SOLUTION at 20° to 25°C (68° to 77°F) with excursions permitted to 15° to 30°C (59° to 86°F).
- The solution may be held for up to 28 days if stored in unopened containers.
- ONCE A CONTAINER IS OPENED, ANY UNUSED SOLUTION MUST BE DISCARDED WITHIN 48 HOURS.
Storage
There is limited information regarding Mafenide Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Mafenide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Mafenide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Mafenide in the drug label.
Precautions with Alcohol
- Alcohol-Mafenide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- MAFENIDE ACETATE®[1]
Look-Alike Drug Names
There is limited information regarding Mafenide Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Mafenide |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Mafenide |Label Name=Mafenide11.png
}}
{{#subobject:
|Label Page=Mafenide |Label Name=Mafenide11.png
}}
