Macimorelin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sonya Gelfand
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Macimorelin is a growth hormone (GH) secretagogue receptor agonist that is FDA approved for the diagnosis of adult growth hormone deficiency. Common adverse reactions include dysgeusia, dizziness, headache, fatigue, nausea, hunger, diarrhea, upper respiratory tract infection, feeling hot, hyperhidrosis, nasopharyngitis, and sinus bradycardia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
- Macimorelin is indicated for the diagnosis of adult growth hormone deficiency (AGHD).
Limitations of Use
- The safety and diagnostic performance of macimorelin have not been established for subjects with a body mass index (BMI) > 40 kg/m2.
Recommended Dose
- The recommended dose is a single oral dose of 0.5 mg/kg of macimorelin. The dose is administered as a reconstituted solution.
Important Recommendations Before macimorelin Use
- Discontinue strong CYP3A4 inducers prior to macimorelin use.
- Discontinue growth hormone (GH) therapy at least one week before administering macimorelin.
- Avoid the use of macimorelin with drugs known to affect pituitary GH secretion.
- For patients with deficiencies in sex hormones, thyroid hormone and/or glucocorticoid, adequately replace each of the missing hormones before administering macimorelin.
- Ensure that the patient has fasted for at least 8 hours before macimorelin use.
Dosage Forms and Strengths
- For oral solution: 60 mg white to off-white granules in a pouch for reconstitution in 120 mL of water, resulting in a solution of 0.5 mg/mL of macimorelin.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding macimorelin Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding macimorelin Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Macimorelin FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding macimorelin Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding macimorelin Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
- None.
Warnings
QT Prolongation
- Macimorelin causes an increase of about 11 msec in the corrected QT (QTc) interval. QT prolongation can lead to development of torsade de pointes-type ventricular tachycardia with the risk increasing as the degree of prolongation increases. The concomitant use of macimorelin with drugs that are known to prolong the QT interval should be avoided.
Potential for False Positive Test Results with Use of Strong CYP3A4 Inducers
- Concomitant use of strong CYP3A4 inducers with macimorelin can decrease macimorelin plasma levels significantly and thereby lead to a false positive result. Strong CYP3A4 inducers should be discontinued and enough time should be given to allow washout of CYP3A4 inducers prior to test administration.
Potential for False Negative Test Results in Recent Onset Hypothalamic Disease
- Adult growth hormone (GH) deficiency caused by a hypothalamic lesion may not be detected early in the disease process. Macimorelin acts downstream from the hypothalamus and macimorelin stimulated release of stored GH reserves from the anterior pituitary could produce a false negative result early when the lesion involves the hypothalamus. Repeat testing may be warranted in this situation.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trial of another drug and may not reflect the rates observed in practice.
- The data in TABLE 1 are derived from an open-label, randomized, cross-over study that compared the diagnostic performance of macimorelin to the insulin tolerance test (ITT) for the diagnosis of adult growth hormone deficiency. A total of 154 subjects with a high to low pre-test probability of having adult growth hormone deficiency received a single oral dose of 0.5 mg/kg macimorelin. Out of 154 subjects, 58% were male, 42% female, and 86% of white origin. Median values were for age 41 years (range: 18 – 66 years) and body mass index was 27.5 kg/m2 (range: 16 – 40 kg/m2). Common adverse reactions presented in TABLE 1 were adverse reactions that were not present at baseline and occurred during macimorelin dosing in at least two individuals.

Postmarketing Experience
There is limited information regarding Macimorelin Postmarketing Experience in the drug label.
Drug Interactions
- Drugs that Prolong QT Interval
- Cytochrome P450 (CYP) 3A4 Inducers
- Drugs Affecting Growth Hormone Release
Drugs that Prolong QT Interval
- Co-administration of macimorelin with drugs that prolong the QT interval (such as antipsychotic medications (e.g., chlorpromazine, haloperidol, thioridazine, ziprasidone), antibiotics (e.g., moxifloxacin), Class 1A (e.g., quinidine, procainamide) and Class III (e.g., amiodarone, sotalol) antiarrhythmic medications or any other medications known to prolong the QT interval) may lead to development of torsade de pointes-type ventricular tachycardia. Avoid concomitant use of macimorelin with drugs that prolong the QT interval. Sufficient washout time of drugs that are known to prolong the QT interval prior to administration of macimorelin is recommended.
Cytochrome P450 (CYP) 3A4 Inducers
- Co-administration of a strong CYP3A4 inducer with macimorelin (e.g., carbamazepine, enzalutamide, mitotane, phenytoin, rifampin, St. John's wort, bosentan, efavirenz, etravirine, modafinil, armodafinil, rufinamide) may reduce the plasma macimorelin concentrations and may lead to false positive test results. Discontinue strong CYP3A4 inducers prior to macimorelin use. Sufficient washout time of strong CYP3A4 inducers prior to administration of macimorelin is recommended.
Drugs Affecting Growth Hormone Release
- The following drugs may impact the accuracy of the macimorelin diagnostic test. Avoid concomitant use of macimorelin with the following:
- Drugs that directly affect the pituitary secretion of growth hormone (such as somatostatin, insulin, glucocorticoids, and cyclooxygenase inhibitors such as aspirin or indomethacin).
- Drugs that may transiently elevate growth hormone concentrations (such as clonidine, levodopa, and insulin).
- Drugs that may blunt the growth hormone response to macimorelin (such as muscarinic antagonists: atropine, anti-thyroid medication: propylthiouracil, and growth hormone products). Discontinue growth hormone products at least one week before administering the macimorelin diagnostic test.
- Sufficient washout time of drugs affecting growth hormone release prior to administration of macimorelin is recommended.
Use in Specific Populations
Pregnancy
Risk Summary
- There are no available data with macimorelin use in pregnant women to inform a drug associated risk for adverse developmental outcomes. Animal reproduction studies have not been conducted with macimorelin. Macimorelin is indicated as a single dose which limits the risk of adverse developmental outcomes from exposure to macimorelin.
- The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 – 4% and 15 – 20%, respectively.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Macimorelin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Macimorelin during labor and delivery.
Nursing Mothers
Risk Summary
- There are no data on the presence of macimorelin in human or animal milk, the effects on the breastfed infant or the effects on milk production. The lack of clinical data during lactation precludes a clear determination of the risk of macimorelin to an infant during lactation; therefore, the developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for macimorelin and any potential adverse effecs on the breastfed infant from macimorelin or the underlying maternal condition.
Pediatric Use
- The safety and efficacy of macimorelin in pediatric patients have not been established.
Geriatic Use
- Growth hormone secretion normally decreases with age. Therefore, elderly subjects might require a lower cut-off point for diagnosis of adult growth hormone deficiency. Clinical studies of macimorelin did not include a sufficient number of subjects aged 65 and over to determine whether elderly patients respond differently from younger subjects.
Gender
There is no FDA guidance on the use of Macimorelin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Macimorelin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Macimorelin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Macimorelin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Macimorelin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Macimorelin in patients who are immunocompromised.
Administration and Monitoring
Administration
Directions for Preparation and Administration
- Prepare and administer by a healthcare professional exactly as follows.
Prepare the Macimorelin solution:
- Weigh the patient in kilograms (i.e., kg).
- Determine the number of macimorelin pouches needed to prepare the dose:
- For a patient weighing up to 120 kg, use 1 pouch.
- For a patient weighing more than 120 kg, use 2 pouches.
- Use a glass or transparent plastic container with graduation in milliliters (i.e., mL) to dissolve the entire contents of the pouch(es) in the appropriate volume of water.
- For 1 pouch dissolve in 120 mL of water (corresponds to 60 mg/120 mL).
- For 2 pouches dissolve in 240 mL of water (corresponds to 120 mg/240 mL).
- Stir the macimorelin solution gently for about 2 to 3 minutes (a small amount of un-dissolved particles will remain). The solution will have a final concentration of 0.5 mg/mL.
- Use the macimorelin solution within 30 minutes after preparation.
- Discard any unused macimorelin solution.
Determine the volume of macimorelin solution needed for the test:
- Determine the recommended dose to be administered by multiplying the patient weight in kilogram by 0.5 mg/kg.
- For example, a 70 kg patient will need a 35 mg dose.
- Determine the volume of prepared macimorelin solution to be administered by dividing the recommended dose by 0.5 mg/mL.
- For example, a patient requiring a dose of 35 mg will need 70 mL of reconstituted macimorelin solution.
- Use a syringe (without a needle) with graduations in mL to measure the exact volume of macimorelin solution to be administered and transfer the required volume of macimorelin solution into a drinking glass.
Administer the macimorelin solution and perform the test:
- Have the patient being tested drink the entire volume of macimorelin solution in the drinking glass (i.e., the dose) within 30 seconds.
- Observe the patient being tested per routine for the duration of the test.
- Draw venous blood samples for GH determination at 30 minutes, 45 minutes, 60 minutes and 90 minutes after administration of macimorelin.
- Prepare serum samples and send to a laboratory for growth hormone determinations.
Interpretation of Macimorelin Test Results
- Clinical studies have established that a maximally stimulated serum GH level of less than 2.8 ng/mL (i.e., at the 30, 45, 60 and 90 minute timepoints) following macimorelin administration confirms the presence of adult growth hormone deficiency.
Monitoring
- Venous blood samples for growth hormone (GH): At 30, 45, 60, and 90 minutes following administration: maximally stimulated serum GH levels of 2.8 nanogram (ng)/mL or less at each interval confirms the presence GH deficiency.
IV Compatibility
There is limited information regarding the compatibility of Macimorelin and IV administrations.
Overdosage
- In the event of an overdose, symptomatic and supportive measures should be employed.
Pharmacology
| |
Macimorelin
| |
| Systematic (IUPAC) name | |
| 2-Amino-N-[(2R)-1-[[(1R)-1-formamido-2-(1H-indol-3-yl)ethyl]amino]-3-1H-indol-3-yl)-1-oxopropan-2-yl]-2-methylpropanamide | |
| Identifiers | |
| CAS number | |
| ATC code | V04 |
| PubChem | ? |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 534.6 g/mol |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
- Macimorelin stimulates GH release by activating growth hormone secretagogue receptors present in the pituitary and hypothalamus.
Structure

Pharmacodynamics
GH stimulation
- Maximum GH levels are observed between 30 to 90 minutes after administration of macimorelin.
Cardiac electrophysiology
- The effects of macimorelin on ECG parameters were investigated in a dedicated Thorough QT study that investigated in a 3-way cross-over design with 60 healthy subjects the effects of a supra-therapeutic dose of macimorelin (2 mg/kg) (4 times the recommended dosage) in comparison with placebo and with moxifloxacin. This study showed a mean baseline- and placebo-adjusted change (upper single-sided 95% confidence interval) in QTcF of 9.6 msec (11.4 msec) at 4 h post-dose, which occurred after the mean maximum macimorelin plasma concentration (0.5 h). A similar increase in the QTcF interval was also observed in a single-ascending dose study, which included three dose levels (0.5 mg/kg, and 1 mg/kg and 2 mg/kg (2 times and 4 times the recommended dosage, respectively). All three doses levels studied showed a similar magnitude of QTcF prolongation in the Thorough QT study, suggesting an absence of dose dependent changes. The mechanism for the observed QTcF prolongation is unknown.
Pharmacokinetics
- The mean plasma macimorelin concentrations are similar between patients with AGHD and healthy subjects for 1.5 hours following administration of a single oral dose of 0.5 mg macimorelin/kg body weight.
Absorption
- The maximum plasma macimorelin concentrations (Cmax) were observed between 0.5 hour and 1.5 hours following oral administration of 0.5 mg macimorelin/kg body weight to patients with AGHD under fasting for at least 8 hours. A liquid meal decreased the macimorelin Cmax and AUC by 55% and 49%, respectively.
Elimination
- An in vitro human liver microsomes study showed that CYP3A4 is the major enzyme to metabolize macimorelin.
- Macimorelin was eliminated with a mean terminal half-life (T1/2) of 4.1 hours following administration of a single oral dose of 0.5 mg macimorelin/kg body weight in healthy subjects.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
- Long-term carcinogenesis studies in rodents have not been conducted.
Mutagenesis
- Macimorelin did not cause mutations in bacteria under assay conditions with or without metabolic activation. There were also no mutations or clastogenic effects in mouse lymphoma cells with or without metabolic activation.
Impairment of Fertility
- No studies have been conducted to assess the effect of macimorelin on fertility.
Clinical Studies
- The diagnostic efficacy of the macimorelin test was established in a randomized, open-label, single-dose, cross-over study. The objective of the study was to compare the level of agreement between macimorelin test results and insulin tolerance test (ITT) results in adult patients with different pre-test probability of growth hormone deficiency and healthy control subjects. The four groups of individuals evaluated were:
- Group A: Adults with a high likelihood of growth hormone deficiency (GHD)
- Structural hypothalamic or pituitary lesions and low insulin-like growth factor 1 (IGF-1), and/or
- Three or more pituitary hormone deficiencies and low IGF-1, or
- Childhood onset GHD with structural lesions and low IGF-1.
- Group B: Adults with an intermediate likelihood of GHD
- Eligible subjects not qualifying for either high or low likelihood.
- Group C: Adults with a low likelihood of GHD
- One risk factor for GHD only, such as history of distant traumatic brain injury or one pituitary hormone deficiency only with otherwise normal pituitary function, or
- Isolated idiopathic childhood onset GHD without additional pituitary deficits.
- Group D: Healthy adult controls
- Healthy subjects matching Group A subjects by sex, age ± 5 years, body mass index (BMI ± 2 kg/m2), and estrogen status (females only).
- For both the ITT and the macimorelin test, serum concentrations of growth hormone were measured at 30, 45, 60, and 90 minutes after drug administration. The test was considered positive (i.e., growth hormone deficiency diagnosed) if the maximum serum GH level observed after stimulation was less than the pre-specified cut point value of 2.8 ng/mL for the macimorelin test or 5.1 ng/mL for the ITT.
- The level of negative and positive agreement between the results of the ITT and the macimorelin test was used to evaluate the performance of the macimorelin test. In the study, the ITT is used as the benchmark (i.e., a negative ITT indicates absence of disease and a positive ITT indicates presence of disease). Negative agreement is the proportion of subjects with a negative ITT (i.e., those who do not have GHD per the ITT) who also have a negative macimorelin test. With a high level of negative agreement, the macimorelin test will not wrongly diagnose an individual without GHD per the ITT as having GHD. Positive agreement is the proportion of subjects with a positive ITT (i.e., those who have GHD per the ITT) who also have a positive macimorelin test. With a high level of positive agreement, the macimorelin test will not wrongly diagnose an individual with GHD per the ITT as not having GHD. The agreement measures are defined mathematically below.

Results
- One hundred and fifty-seven subjects underwent at least one of the two tests in this study, 59% were male, 41% female, and 86% of white origin. The median age was 41 years (range: 18 – 66 years) and body mass index 27.5 kg/m2 (range: 16 – 40 kg/m2). The study relied on a cross-over design and each participant was to undergo the two diagnostic tests and serve as his or her own control. Data on both tests were available for 140 subjects; 38 (27%) in Group A, 37 (26%) in Group B, 40 (29%) in Group C, and 25 (18%) in Group D. One out of 154 macimorelin tests (0.6%) performed failed due to a technical error and 27 out of 157 ITTs (17.2%) performed failed because induction of severe hypoglycemia (i.e., the stimulus) could not be achieved.
- Two-by-two tables presenting the pre-specified primary analysis results for the ITT and macimorelin test are shown below for all subjects (Groups A, B, C, and D combined) and for each group separately. The estimates for negative and positive agreement between macimorelin and the ITT in the overall study population were 94% and 74% with lower 95% confidence interval bounds 85% and 63%, respectively. Negative and positive agreement between macimorelin and the ITT in subjects with intermediate or low risk (Groups B and C) were 93% and 61% with lower 95% confidence interval bounds 80% and 43%, respectively. These results are based on peak GH values (maximum GH concentrations across all measurement timepoints).
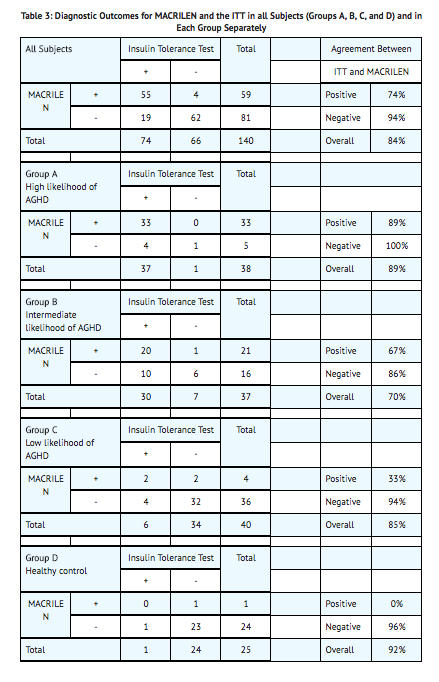
- Repeatability was tested in a subset of 34 subjects who underwent two macimorelin tests. Agreement between the result of the first test and the second test was observed in 31 cases (91.2%).
How Supplied
- Macimorelin 60 mg is supplied as white to off-white granules in an aluminum pouch. Each pouch contains 60 mg macimorelin (equivalent to 68 mg macimorelin acetate) that when reconstituted with 120 mL of water provides a 60 mg/120 mL (0.5 mg/mL) macimorelin solution.
- Macimorelin is available in boxes containing 1 pouch per box (NDC 71090-002-02).
- Before administration, macimorelin for oral solution must be reconstituted by a healthcare professional.
Storage
- Store pouches under refrigeration at 2-8°C (36-46°F).
- The solution must be used within 30 minutes after preparation. Discard unused portion.
Images
Drug Images
{{#ask: Page Name::Macimorelin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Macimorelin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Instruct patients to discontinue treatment with GH at least one week before administering macimorelin. Also, instruct patients to discontinue other medications that may interfere with the diagnostic test results prior to macimorelin administration.
- Instruct patients to fast for at least 8 hours before macimorelin administration
Precautions with Alcohol
Alcohol-Macimorelin interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Macrilen
Look-Alike Drug Names
There is limited information regarding Macimorelin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.